Changes in the intracellular free Ca2+ concentration [Ca2+] in regulate a large number of physiological processes and have a major impact on cell development and function. The identification of STIM, the endoplasmic reticulum (ER) Ca2+ sensor, and Orai, a key subunit of the Ca2+ release-activated Ca2+ (CRAC) channel pore, have provided the tools to illuminate the mechanisms of CRAC channel regulation that play an important role in modulating the free Ca2+ concentration in non-excitable cells. Store-operated Ca2+ entry (SOCE) is a common and ubiquitous mechanism for regulating Ca2+ influx into cells and is widely expressed in most tissues. Alomone Labs offers primary polyclonal antibodies, belonging to Orai and STIM family that can be used in a variety of research disciplines, as described and presented in the article below.
Introduction
Depletion of Ca2+ from the ER or other intracellular Ca2+ stores is followed by Ca2+ influx across the plasma membrane. This Ca2+ entry pathway is mediated via activation of specific plasma membrane channels named store-operated Ca2+ entry (SOCE) channels or Ca2+ release-activated Ca2+ (CRAC) channels. It was suggested that the release and entry of Ca2+ across the plasma membrane might be linked by a common mechanism, based on the kinetics of refilling intracellular Ca2+ pools following their depletion10. The molecular identity of the channel components and the mechanism by which the status of Ca2+ in the ER is transmitted to the plasma membrane, has been unknown for many years. In 2005, STIM1 (stromal interaction molecule 1) was identified as the mammalian ER Ca2+ sensor11,18, followed closely by the identification of Orai1 (also known as CRACM1) a key subunit of the CRAC channel pore7,20,24. STIM1 contains 685 amino acids with a single transmembrane domain with several functional domains including a luminal EF-hand motif that senses ER Ca2+ concentration and multiple protein-protein interaction motifs. Mammalian cells express an additional STIM protein, designated STIM2. STIMs are expressed in nearly all mammalian tissues and are conserved from Drosophila to humans and are mainly localized in the ER and plasma membrane.
The second protein Orai (named after mythological characters that served as gate keepers), has four transmembrane domains 7,10,21. CRAC channels are formed by the homomeric assembly of four Orai1 subunits9,12,14.
The structure of Orai is not homologous to any known channel. This is perhaps not surprising, given that these channels are distinguished by an extremely high selectivity for Ca2+ and low conductance compared with all other known channels. Mammalian cells express three closely related homologues, Orai1, Orai2 and Orai3. All three isoforms appear to function similarly in producing store-operated Ca2+ entry15.
Recently, it has been proposed that STIM and Orai underlie the welldescribed Ca2+– release-activated current (ICRAC) in non-excitable cells16,23. The discovery of these proteins has propelled rapid progress in illuminating the molecular mechanisms of this pathway and its function in various tissues.
Upon Ca2+ depletion in the ER, Ca2+-sensing STIM1 proteins translocate to close proximity of the plasma membrane, where they aggregate into multiple puncta. Strikingly, Orai1 molecules also translocate to the same STIM1-containing structures upon store depletion, where they open to mediate Ca2+ influx4,17,22.
Whole-cell ICRAC current generates little visible current noise and the activity of single CRAC channels is yet to be directly measured. The characteristic properties of ICRAC include a lack of voltage-dependent gating, an inwardly rectifying current-voltage relationship, and blockade by Ni2+, La3+ and Cd2+ 15.
Several reports suggest different store-operated Ca2+ channels in different cell types. Prior to STIM and Orai recognition as key components of the ICRAC current in mast cells, the transient receptor potential channel (TRPC) family was the proposed candidate for store-operated channel15. Other possibilities for SOCE include complex formation between TRPC and STIM or a triad between TRPC, Orai, and STIM. Thus, it has been suggested that Orai and STIM proteins might serve multiple functions and display very different biophysical properties in different cell types depending on the molecular composition of the channel complexes. Orai/STIM channel complexes might consist of not only different Orais and STIMs but also other channel subunits5.
Orai and STIM in Inflammatory Processes
SOCE is known to be a critical event for NADPH oxidase (NOX2) regulation in neutrophils. Defective NOX2 activity has been linked to various inflammatory diseases. Activation of NOX2 depends on Ca2+ influx leading to the hypothesis that STIM1 inhibition might contribute to a reduction in NOX2 activity (H2O2 production). Stimulation of differentiated HL-60 cells (Human promyelocytic leukemia cells) with fMLF (a bacterial peptide), caused a rapid [Ca2+] in increase from intracellular stores and activated NOX2 a few seconds later, resembling to thapsigargin-induced release of Ca2+ from intracellular stores (by inhibiting Ca2+-ATPase), thereby triggering SOCE Ca2+ influx. The fact that SOCE was triggered upon the activation of these cells, leads to the possibility that perhaps STIM proteins are involved in the process. In order to verify this possibility, expression of STIM proteins was checked. It was found that STIM1 proteins are expressed in HL-60 cells3. Anti-STIM1 (extracellular) Antibody (#ACC-063) was used in indirect immunofluorescence technique and analyzed by flow cytometry. The level of STIM1 protein was higher in differentiated HL-60 cells than in undifferentiated cells whereas the level of STIM2 protein remained unchanged during the differentiation process. To address the issue whether STIM1 and STIM2 are functionally involved in the SOCE pathway in phagocytic cells, small interfering RNA (siRNA) was used to target and selectively suppress both endogenous STIM1 and STIM2 proteins. The ability of each siRNA to inhibit protein expression was evaluated by western blot analysis. Anti-STIM1 (extracellular) antibody demonstrated that STIM1 protein levels were significantly reduced by STIM1 siRNA. On the other hand, STIM2 siRNA did not alter STIM1 protein levels confirming the specificity of STIM1 and STIM2 siRNAs. The effects of STIM1 and STIM2 knockdown on Ca2+ mobilization and moreover whether their regulation could have a potential impact on the control of inflammatory processes through a decrease of reactive oxygen species production were studied. As expected, STIM1 siRNA caused suppression of fMLF-mediated [Ca2+] in elevation by more than 50%. The Ca2+ influx observed following thapsigargin stimulation was abolished by approximately 50% after siRNA treatment, confirming the involvement of STIM1 in the SOCE pathway. STIM2 seems to not be involved in the process as siRNA treatment had no transverse effects in SOCE. These results point out the importance of STIM1 signaling for the correct sequence of NOX2 activation3.
Orai1 is expressed in human polymorphonuclear neutrophils (PMNs) and in HL-60 cells19. Orai1 was knocked down in order to examine whether SOCE mediated by Orai1 is responsible for the transition from arrest to shape polarization and migration in the acute response to inflammation. A knockdown of Orai1 expression was done using siRNA in human PMNs or in differentiated HL-60 and in mice heterozygous for Orai1 gene deletion (Orai1-/+). Expression of Orai1 in siRNA treated cells using Anti-Human Orai1 (extracellular) antibody (#ACC-060) in fluorescence flow cytometry shows that siRNA reduced Orai1 expression by approximately 50% compared to scramble siRNA treated cells (Figure 1). In the study, Ca2+ entry was significantly reduced when Orai1 function was impaired by heterozygous knockout in a mouse model or by siRNA knockdown in HL-60 cells. Reduced Orai1 expression correlated with the delayed onset of arrest and reduced ability to the transition of a polarized migratory phenotype19.
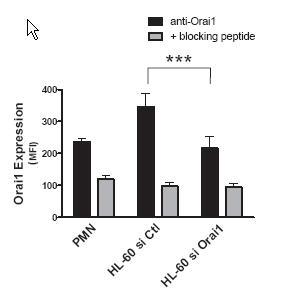
Isolated mouse and human PMNs, or 2-day differentiated HL-60 were assessed for protein expression using Anti-Human Orai1 (extracellular) antibody (#ACC-060) and quantified by a labeled secondary antibody using fluorescence flow cytometry. Protein expression levels were measured from samples of HL-60 cells transfected with negative control or Orai1-specific siRNA (n = 10) and PMNs (n = 4). Background fluorescence resulting from nonspecific antibody binding was measured by analyzing cells treated with the negative control antigen.
Adapted from reference 19 with permission of The American Society of Hematology.
Orai and STIM in the Cardiovascular System
Store-operated Ca2+ entry (SOCE) through TRPCs is important in the development of cardiac hypertrophy. Recently, STIM1 was identified as a key regulator of SOCE. The role of STIM1 in the development of cardiomyocyte hypertrophy was investigated. Western blot analysis was used to analyze the expression of STIM1 and revealed that there are no differences in the STIM1 expression level between normal hearts and the hypertrophied hearts of abdominal aortic banded (AAB) rats, a typical animal model of cardiac hypertrophy13(Figure 2A). TRPC1 expression using Anti-TRPC1 antibody (#ACC-010) was significantly increased in rats subjected to AAB whereas TRPC5 and TRPC6 expression was unchanged (Figure 2B) using Anti-TRPC5 (#ACC-020) and Anti-TRPC6 (#ACC-017) antibodies. Conversely knockdown of STIM1 prevented the induction of hypertrophic responses in cardiomyocytes. These results suggest that STIM1 plays an essential role in the development of cardiomyocyte hypertrophy. It is thought that protein level of the endogenous STIM1 is sufficient to facilitate hypertrophic responses without its upregulation. In the same cardiomyocytes, changes in [Ca2+]in were measured based on the fluorescence ratio of Fura-2AM. SOCE was significantly decreased in siSTIM1 treated cells. Nuclear factor of activated T cells (NFAT) is a downstream signaling event following sustained Ca2+ entry. In cardiomyocyte hypertrophy, NFAT activity was measured by EGFP fluorescence. Cardiomyocytes in which STIM1 was knocked down showed suppressed NFAT activation, indicating the importance of STIM1 in eliciting NFAT activity. Overall, these data suggest the involvement of STIM1 in regulating SOCE and NFAT in cardiomyocytes13.
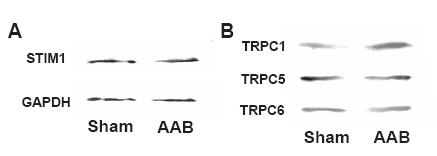
A) Marginal changes in STIM1 were detected in hypertrophied heart compared with normal heart using Anti-STIM1 (extracellular) antibody (#ACC-063) in western blot analysis. B) TRPC1 expression using Anti-TRPC1 antibody (#ACC-010) was significantly increased in rats subjected to AAB (whereas TRPC5 and TRPC6 expression was unchanged using Anti-TRPC5 (#ACC-020) and Anti-TRPC6 (#ACC-017) antibodies. Each experiment was repeated three times with the same results.
Adapted from reference 13 with permission of Elsevier.
Platelet-derived growth factor (PDGF) is an important promigratory agonist of vascular smooth muscle cells (VSMC) with an established role in vascular disease. Inhibitors of store-operated Ca2+ entry (Gd3+ and 2 aminoethoxydiphenyl borate) prevent PDGF-mediated Ca2+ entry in cultured rat aortic VSMC. PDGF-induced Ca2+ entry was substantially inhibited by the knockdown of Orai1, STIM1, and PDGFRβ, whereas knockdown of Orai2, Orai3, TRPC1, TRPC4, and TRPC6 had no significant effect, establishing STIM1/Orai1 as the Ca2+ entry route activated in response to PDGFRβ stimulation in VSMC. As shown in Figure 3A, Anti-Human Orai1 (extracellular) antibody recognized a single band which decreased 72 h after transfection with specific siRNA against Orai1. Additional experiments further validated the use of Anti-Human Orai1 (extracellular) antibody in immunofluorescence studies. A similar Orai1 knockdown approach followed by immunofluorescence staining with Anti-Human Orai1 (extracellular) in cultured synthetic VSMC, is depicted in Figure 3B. A dramatic decrease in Orai1 staining 72 h after treatment with siRNA against Orai1 is clearly demonstrated. Knockdown of STIM1 or Orai1 inhibited VSMC migration in vitro in response to PDGF, whereas STIM2, Orai2, and Orai3 silencing had no significant effect. The results establish that STIM1 and Orai1 are important components for PDGF-mediated Ca2+ entry and migration in VSMC. As shown in Figure 3C, Orai1 staining colocalized with germ agglutinin (a well-known plasma membrane marker), suggesting plasma membrane localization of Orai1. Protein levels of STIM1 and Orai1 were also upregulated in VSMC from injured carotid arteries compared with noninjured vessels2.
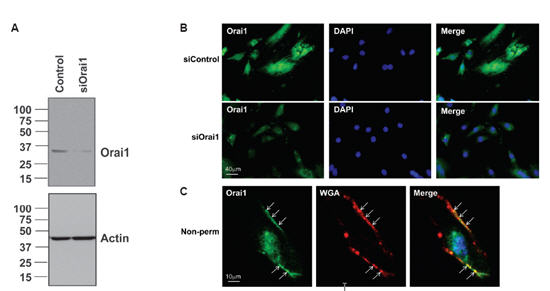
A) Western blot analysis showing Orai1 expression using Anti-Human Orai1 (extracellular)antibody (#ACC-060) following siRNA transfection. B) VSMC were transfected with nontargeting siRNA (siControl) or siRNA targeting Orai1 (siOrai1), fixed, and processed for immunofluorescence under permeabilized conditions using Anti-Human Orai1 (extracellular); DAPI was used as a nuclear marker. A reduction in Orai1 expression is clearly detected in siOrai1 images. C) VSMC were fixed and processed for immunofluorescence under nonpermeabilized conditions using Anti-Human Orai1 (extracellular), wheat germ agglutinin (WGA)-Alexa Fluor 594 was used as a plasma membrane marker and DAPI as a nuclear marker. Arrows indicate colocalization between Orai1 and WGA at the plasma membrane (Merge, yellow staining).
Adapted from reference 2 with permission of The American Physiological Society.
Orai and STIM in other Systems
The molecular basis of SOCE activation involving TRPC1, STIM1 and Orai1 using Fura-2AM fluorescence was studied in HEK293 cells. Expression of Orai1-STIM1 in HEK293 cells resulted in activation of typical inwardly rectifying ICRAC-like currents in response to thapsigargin stimulation. On the other hand, expression of TRPC channels with STIM1 also generated SOCE channels, displaying linear currents following thapsigargin stimulation. The study shows that TRPC1-STIM1 generate SOCE channels that are distinct from CRAC channels (generated by Orai1-STIM1) in the property of their currents. However, TRPC1-STIM1-induced ISOCE was significantly reduced by the knockdown of endogenous Orai1 in HEK293 cells. Thus, TRPC1-STIM1-dependent SOC channel function also requires endogenous Orai1. The decrease in Orai1 protein levels was measured by immunoprecipitation with Anti-Human Orai1 (extracellular) antibody. Figure 4 shows Orai1 in cells treated for 48 h with control siRNA and with increasing amounts of siRNA targeted against Orai15.
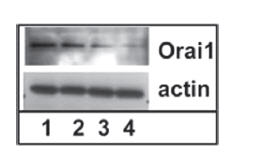
Cells were treated with siRNAs for 48 h. The effect of siOrai1 on expression of Orai1 is shown by western blot using Anti-Human Orai1 (extracellular) antibody (#ACC-060). Lane 1 shows sample from cells treated with control siRNA, lanes 2, 3, and 4, correspond to samples from cells treated with 0.2, 0.4, or 0.8 nmol of siOrai1, respectively. Actin is used as a loading control.
Adapted from reference 5 with permission of American Society for Biochemistry and Molecular Biology.
Glial cells (Human astrocytoma U373 MG) respond to exogenously applied histamine with transient increase in [Ca2+]in followed by Ca2+ entry across the cell membrane. This action is mediated by the activation of H1 receptors, expressed in high density in the plasma membrane of U373 MG cells. U373 MG cells express TRPC1, 4,- 5 and 6 (shown using Anti-TRPC1, Anti-TRPC4 (#ACC-018), Anti-TRPC5 and Anti-TRPC6 antibodies). In addition to TRPC proteins, expression of STIM1 and Orai1 (using Anti-Human Orai1 (extracellular)) subunits was also detected in immunocytochemistry studies (Figure 5). The finding that STIM1 and Orai1 are expressed in these cells opens the interesting possibility that histamine might induce Ca2+ entry through more than one species of SOC in U373 MG cells1.

Confocal indirect immunofluorescence images obtained from cells labeled with specific Anti-Human Orai1 (extracellular) antibody (#ACC-060) (A). Green and red signals are derived from fluorescein and nuclear staining with propidium iodide, respectively. Negative controls consisted in incubating of the samples with Anti-Human Orai1 (extracellular) antibody preincubated with its negative control antigen (B).
Adapted from reference 1 with permission of John Wiley & Sons Ltd.
Orai and STIM in Metabolic Syndromes
Histamine-induced divalent cation influx was demonstrated in isolated Ossabaw miniature pig adrenal chromaffin cells. This current correlated, with the expression level of TRPCs, namely TRPC5, detected in pig adrenal medulla using Anti-TRPC5 antibody. Orai1/STIM1 proteins, mediating store-operated Ca2+ influx were also expressed in the adrenal medulla as shown using Anti-Human Orai1 (extracellular) and Anti-STIM1 (extracellular) antibodies. However, the amounts of Orai1/STIM1 proteins were not significantly altered in the metabolic syndrome adrenal medulla (Figure 6A). On the other hand, the TRPC5 protein levels increased about 3- to 4-fold (Figure 6B)8.

A) Western blot analysis with Anti-Human Orai1 (extracellular) antibody (#ACC-060) and Anti-STIM1 (extracellular) antibody (#ACC-063) on extracts from the adrenal medulla isolated from lean (L) and metabolic syndrome (M) pigs. The graph is a quantification of Orai1 and Stim1 protein in lean (L, n = 8) vs. metabolic syndrome (M, n = 7) pig adrenal medulla (normalized to β-actin). B) Western blots with Anti-TRPC5 antibody (#ACC-020) from same extracts as in A normalized to β-actin as shown in the graph. *, Significant difference between the tested groups (P ≤0.05; n = 4).
Adapted from reference 8 with permission of The Endocrine Society.
In a follow up study, male Ossabaw swines were assigned to diet groups for 54 weeks. The lean control group, were fed a lean standard diet. The metabolic syndrome (MetS) group and the metabolic syndrome exercise trained group, XMetS, were fed a high fat/2% cholesterol atherogenic diet. Increase in SOCE was shown in the coronary smooth muscle cells in the MetS group compared with the lean control group. Changes in the molecular expression of STIM1 and Orai1 were monitored using their respective Alomone Labs antibodies in coronary smooth muscle cells. The immunoblot experiments showed that the MetS group expressed greater STIM1 protein compared to the lean group. The rise in STIM1 protein in MetS was abolished in XMetS compared to lean (Figure 7), whereas Orai1 protein expression showed no overall statistical difference between the three groups. The data support the hypothesis that Ossabaw swine with MetS have increased SOCE and exercise attenuates these events. Further, increased STIM1 protein expression was attenuated by exercise6.
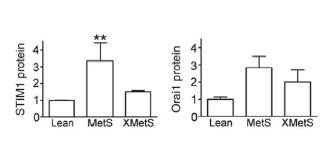
Normalized immunoblots using Anti-STIM1 (extracellular) (#ACC-063), Anti-Human Orai1 (extracellular) (#ACC-060) antibodies and further normalized to β-actin, in Lean MetS and XMetS groups. Increased STIM1 in MetS is abolished by exercise (A) while Orai1 protein expression does not fluctuate between the three groups(B) (ANOVA P = 0.10).
Adapted from reference 6 with permission of Oxford University Press.
References
- Barajas, M. et al. (2008) J. Neurosci. Res. 86, 3456.
- Bisaillon, J.M. et al. (2010) Am. J. Physiol. 298, C993.
- Brechard, S. et al. (2009) Biochem. Pharmacol. 78, 504.
- Cahalan, M.D. et al. (2007) Cell Calcium. 42, 133.
- Cheng, K.T. et al. (2008) J. Biol. Chem. 283, 12935.
- Edwards, J.M. et al. (2010) Cardiovasc. Res. 85, 631.
- Feske, S. et al. (2006) Nature 441, 179.
- Hu, G. et al. (2009) Mol. Endocrinol. 23, 689.
- Ji, W. et al. (2008) Proc. Natl. Acad. Sci. U. S. A. 105, 13668.
- Lewis, R.S. (2001) Annu. Rev. Immunol. 19, 497.
- Liou, J. et al. (2005) Curr. Biol. 15, 1235.
- Mignen, O. et al. (2008) J. Physiol. 586, 419.
- Ohba, T. et al. (2009) Biochem. Biophys. Res. Commun. 389, 172.
- Penna, A. et al. (2008) Nature 456, 116.
- Prakriya, M. (2009) Immunol. Rev. 231, 88.
- Prakriya, M. et al. (2006) Nature 443, 230.
- Putney, J.W., Jr. (2007) Cell Calcium 42, 103.
- Roos, J. et al. (2005) J. Cell Biol. 169, 435.
- Schaff, U.Y. et al. (2010) Blood 115, 657.
- Vig, M. et al. (2006) Curr. Biol. 16, 2073.
- Wissenbach, U. et al. (2007) Cell Calcium 42, 439.
- Wu, M.M. et al. (2007) Cell Calcium 42, 163.
- Yeromin, A.V. et al. (2006) Nature 443, 226.
- Zhang, S.L. et al. (2006) Proc. Natl. Acad. Sci. U. S. A. 103, 9357.