Introduction
GABA (g-aminobutiric acid) is the major inhibitory neurotransmitter in the brain. Its production, release, reuptake and metabolism occur in the nervous system.1
The GABA transmitter interacts with two major types of receptors: ionotropic GABAA (GABAAR) and the metabotropic GABAB receptors. The GABAAR belong to the ligand-gated ion channel superfamily.2
Structure and Function of GABAAR
Like the nAchR, 5HTR and the glycine receptor, the GABAAR, is a heteropentamer, with all of its five subunits contributing to the pore formation. To date, eight subunit isoforms were cloned: α, β, γ, δ, ε, π, φ and ρ1.
In most cases, multiple subtypes within each of these isoforms have been described: six α-subunits, three β-subunits, three γ-subunits, one δ-subunit, one ε-subunit, one π-subunit, one α-subunit and three ρ-subunits (the ρ-subunits actually create a related receptor named GABAcR which is composed of single or multiple ρ-subunits)1,3. The native GABAA receptor, in most cases, consists of 2α, 2β and 1γ subunit. The δ, ε, π and φ are able to substitute for the γ-subunit1.
The regulation of the α2-subunit gene is a very complex process: six α2 spliced isoforms were identified and three promoter regions were defined4.
The binding of GABA to its GABAA receptor results in conformational changes that open a Cl– channel, producing an increase in membrane conductance, resulting in inhibition of neural activity2,3. Since GABAA receptors are a key element in synaptic inhibition, they become a primary target for a wide range of clinical drugs, among them antiepileptic agents, anxiolytic agents, sedatives and anesthetics. Exploring the way GABAAR functions in response to drugs and in diseases might be of great importance for targeting this receptor and for preventing side effects.
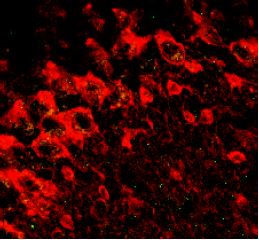
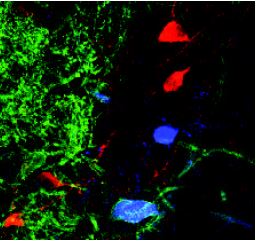
Contributed by W. Hartig and J. Grosche, Leipzig University.
GABAAR in Development and Disease
The genes encoding the GABAAR subunits are differentially expressed depending on both developmental stage and tissue type3,5.
The α subunit is the most common subunit and is expressed ubiquitously. It determines the affinities for allosteric ligands shown by GABAAR. The β-subunit enhances functional channel expression and is an essential component of the GABAAR, while the γ-subunit is responsible for the benzodiazepine sensitivity, which is a property of several types of native receptors and for enhancing the single-channel conductance and open probability3. A number of diseases are associated with the GABAA channels such as temporal lobe epilepsy and Angelman syndrome4,6.
The α2-subunit mRNA occurs very early in rat brain development and declines in adulthood. The failure to complete the normal transition between the α-subunits that are highly expressed in early development (α2, α3, α5) to those expressed in adulthood (α1) might play a major role in the development of temporal lobe epilepsy4,6.
Involvement of GABAAR β3-subunit in Angelman syndrome was also described.
The data suggests that deletion of GABAAR β3- subunit may contribute to the pathogenesis of Angelman syndrome (characterized by severe mental retardation, absence of speech and seizures) without excluding participation of other genes within the region in the process3.
The ability to distinguish between the expression of the different subunits in each developmental stage or disease is very important both to research and diagnosis.
References
- Owens, D.F. et al. (2002) Nature Rev. 3, 715.
- Fuchs, K. et al. (1999) Neurochem. Inter. 34, 387.
- Ashcroft , F.M. (2000) Ion Channels and Disease, Ed 1.
- Fuchs, K. et al. (2002) J. Neurochem. 82, 1512.
- Luddens, H. et al. (1995) Neuropharmacology 34, 245.
- Poulter, M.O. et al. (1999) J. Neurosci. 19, 4654