The p75 neurotrophin receptor (p75NTR) could bind a variety of proteins all with biological significance. While this receptor has no catalytic activities, and therefore influences cellular outcomes through protein-protein interactions, how it controls one cellular event over another remains to be deciphered. Alomone Labs offers a broad range of p75NTR-related products, ranging from monoclonal to both fluorescently labeled monoclonal and polyclonal antibodies. This report aims at illustrating how these products have influenced p75NTR-related research.
Introduction
Neurotrophins display different binding specificities for the Trk receptor family and all promiscuously bind p75NTR resulting in either cell survival or death. p75NTR could also be activated by non-neurotrophic proteins such as pathogens2 as well as pro-neurotrophins12. Evidence shows that the cellular output of p75NTR signaling may depend on the processing event it undertakes.
Processing of p75NTR and Signaling
As opposed to the Trk kinase receptor family, p75NTR has no catalytic activity and therefore instigates downstream activities by binding to adaptor proteins via its intracellular domain. p75NTR activity could also be mediated by the proteolysis of its extracellular domain (a process termed ectodomain shedding). The detachment of the ectodomain, which could be induced by PKC activators, can release proteins from their membrane bound precursors, or down-regulate receptors and other proteins from the cell surface6. TACE (an α-convertase) is involved in the extracellular domain shedding of p75NTR 18 and its action is essential for the following intramembrane cleavage by the γ-secretase complex7, for which its activity has been shown to be dependent on lipid rafts16. Research using rat brain nerve endings indicates that cholesterol (present in lipid rafts) is responsible for keeping a p75NTR pool segregated from the α-secretase3. An experiment where cholesterol was depleted, p75NTR shedding was increased as was shown by western blot analysis using Anti-p75 NGF Receptor (extracellular) Antibody (#ANT-007) on supernatants3 (which did not contain any cells) (Figure 1). These and other data suggest that p75NTR could be proteolyzed when it is located outside lipid rafts due to its accessibility to TACE, while lipid rafts would prevent p75NTR shedding by TACE3. This may suggest a mechanism where unwanted initial processing of p75NTR pools are kept in lipid rafts, and initially processed p75NTR could be transferred to lipid rafts for additional modification by γ-secretase.
Necdin, a protein highly expressed in NGF-dependent neurons has been shown to bind p75NTR and modulate NGF signaling10,14,15. Necdin and p75NTR interaction was first found in a yeast protein-protein interaction screening system, in which part of the data showed that using Mouse Anti-Rat p75NTR (extracellular) Antibody, necdin is co-immunoprecipitated15. Indeed, in NGF-treated PC12 cells, endogenously expressing TrkA and p75NTR, it was observed that using the Mouse Anti-Rat p75NTR (extracellular) Antibody the two receptors are extensively co-immunoprecipitated when necdin is ectopically expressed in these cells11 (Figure 2) strongly suggesting that NGF signaling is enhanced upon necdin expression.

Synaptosomes were treated with mβCD (a cholesterol extracting drug), or with TPA (an activator of p75NTR ectodomain shedding) previous to solubilization with detergent, and subsequently fractionated in a discontinuous sucrose gradient. Supernatants were collected after elimination of the synaptosomes by centrifugation. The same supernatant volumes were immunoblotted using Anti-p75 NGF Receptor (extracellular) Antibody (#ANT-007). Treatment with mβCD yielded a soluble peptide of ~45 kDa, corresponding to the released p75NTR ectodomain.
Adapted from reference 3 with permission of Elsevier.
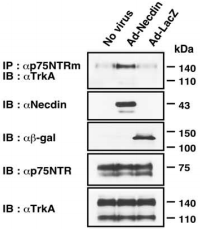
Co-immunoprecipitation assay for the association between TrkA and p75NTR. PC12 cells infected with recombinant adenoviruses expressing necdin (Ad-Necdin) and β-galactosidase (Ad-LacZ) were treated with NGF for 38 hours. Cell lysates were immunoprecipitated (IP) using Mouse Anti-Rat p75 NGF Receptor (extracellular) Antibody (#AN-170) and immunoblotted (IB) with anti-TrkA antibody (upper panel). Expressed proteins in cell lysates are shown in the lower panels.
Adapted from reference 11 with permission of The Society for Neuroscience.
p75NTR Retrograde Trafficking
As, well established, the Nerve Growth Factor (NGF) family of neurotrophins binds the TrkA receptor tyrosine kinase and p75NTR to which it binds with lesser affinity. The NGF-TrkA complex is known to undergo rapid internalization and retrograde trafficking in numerous systems thereby activating signal transduction pathways4,5. While much work has been dedicated towards the understanding and the deciphering of this retrograde signaling mechanism of TrkA, the internalization and trafficking of the neurotrophin p75NTR complex are not well understood. The fact that p75NTR lacks enzymatic activities led Bronfman et al.1 to initiate a study by directly examining the ligand-induced internalization of p75NTR although it has been observed in glial cells9 . Using PC12 cell lines, the authors found that incubation with monoclonal Mouse Anti-Rat p75 NGF Receptor (extracellular)-FITC Antibody (#AN-170-F) in the presence of Native mouse NGF 2.5S protein (99%) or Native mouse NGF 2.5S protein (>95%) (#N-240 or #N-100 respectively), induced the internalization of the ligand-receptor complex after 90 minutes as opposed to a 15 minutes internalization rate for transferrin under the same conditions1 (Figure 3). The study goes on to show that the internalization of p75NTR is independent of TrkA. As endocytosis can occur through the well-defined clathrin-dependent pathway, where entering molecules are delivered to early endosomes and then trafficked to late endosomes and lysosomes for degradation, the route taken by p75NTR internalization was monitored. It was determined by known markers and their internalization pathways that p75NTR internalization occurs via clathrin-coated pits and sorted to early endosomes. When comparing the internalization of both NGF (biotinylated) and p75NTR, extensive colocalization was observed1 (Figure 4). However, following a relatively short internalization period, only NGF positive vesicles could be observed (Figure 4A, upper panels), suggesting that at this time point internalization of NGF-TrkA complex occurs. One hour after the beginning of the internalization process, both NGF and p75NTR could be seen together (Figures 4A lower panel, B and C).
Colocalization of both proteins could also be observed in neurites and growth cones of the differentiated cells1 . Further supporting data suggest that the internalized neurotrophin p75NTR complex possesses signaling ability in neuronal cells once incorporated in endosomes. Recently, p75NTR activation by neurotrophins and its processing was yet again investigated but in the context of ectodomain shedding with internalization of the receptor17. p75NTR ectodomain shedding was demonstrated in PC12 cells treated with PMA (inducer of p75NTR ectodomain shedding) which resulted in a reduction of nearly 50% of cell surface associated p75NTR using Mouse Anti-Rat p75 NGF Receptor (extracellular) Antibody (#AN-170)17 (Figures 5A and B). p75NTR internalization was then tested by measuring the cell surface and intracellular pool of p75NTR before and after permeabilization in control and PMA-treated cells. PMA induced a 5-fold increase in p75NTR internalization compared with basal levels (Figures 5C and D). However, when cells were treated with NGF (purchased from Alomone Labs), the level of p75NTR internalization increased 3-fold compared with PMA treatment17. These results suggest that the trafficking of p75NTR, in addition to being regulated by NGF binding is also regulated by PKC (through PMA treatment) in a NGF-dependent manner. Retrograde transport of p75NTR was also observed in the superior cervical8 ganglion and to a lesser extent in the trigeminal ganglion using Anti-Rat p75NTR-FITC antibody13.
In conclusion, the different means of processing activated p75NTR, whether it be protein-protein interactions with adaptor proteins, ectodomain shedding or retrograde signaling, certainly have influences on the cellular outcome.
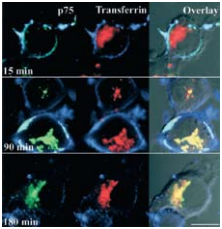
Visualization of the internalization of p75NTR labeled with 3 μg/ml Mouse Anti-Rat p75 NGF Receptor (extracellular)-FITC Antibody (#AN-170-F) (green) in the presence of 20 nM Native mouse NGF 2.5S protein (99%) or Native mouse NGF 2.5S protein (>95%) (#N‑240 or #N-100 respectively) and immunolabeled transferrin by confocal microscopy on PC12 cells. After incubation, cells were fixed and incubated with anti-mouse RRX (blue) to label cell-surface p75NTR.
Adapted from reference 1 with permission of The Society for Neuroscience.
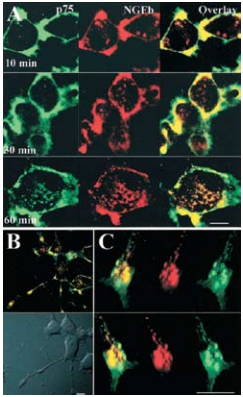
A) Confocal microscopy on cell bodies of differentiated PC12 cells treated with 3 μg/ml Mouse Anti-Rat p75 NGF Receptor (extracellular)-FITC Antibody (#AN-170-F) (green) and 20 nM Native mouse NGF 2.5S protein (99%) or Native mouse NGF 2.5S protein (>95%) (#N-240 or #N-100 respectively) labeled with biotin for 90 minutes at 4°C, washed and incubated for 30-45 minutes at 4°C with strepavidin-Alexa-647, and then incubated for the indicated times at 37°C. Scale bar, 10 μm. B) Merged confocal images of differentiated PC12 cells treated as described above and incubated for 60 minutes at 37°C. Scale bar, 10 μm. C) Confocal three dimensional reconstruction of serial z-planes of a growth cone of differentiated PC12 cell treated as described above. Different rotation angles are shown. Scale bar, 5 μm.
Adapted from reference 1 with permission of The Society for Neuroscience.

Immunocytochemical staining of sympathetic neuronal cultures with Mouse Anti-Rat p75 NGF Receptor (extracellular) Antibody (#AN-170). Cells were treated in vivo with Mouse Anti-Rat p75 NGF Receptor (extracellular) Antibody together with Recombinant human BDNF protein (#B-250) to induce p75NTR internalization. Neurons were then fixed, permeabilized and treated with anti-MAP1b, followed by secondary antibodies recognizing anti-MAP1b (blue) and Mouse Anti-Rat p75 NGF Receptor (extracellular) Antibody (green).
This figure was kindly provided by Francisca C Bronfman, Associate Professor from the Department of Physiology Neurobiology Unit, Faculty of Biological Sciences Pontificia Universidad Catolica de Chile.
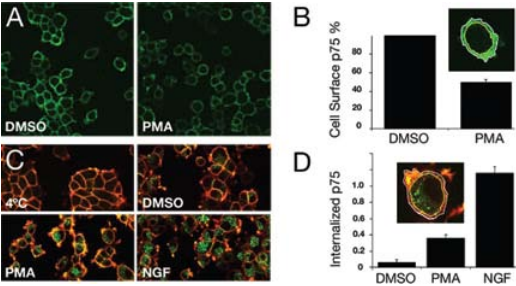
A) Cell surface localization of p75NTR in PC12 cells. PC12 cells were incubated with DMSO or PMA for 1 hour at 37°C, cooled to 4°C, and subsequently incubated for 1 hour with Mouse Anti-Rat p75 NGF Receptor (extracellular) Antibody (#AN-170), recognizing the ectodomain of p75NTR. After fixation, cells were immunolabeled with donkey anti-mouse Alexa-488 (green). A clear decrease in immunoreactivity was observed upon PMA treatment. B) Quantification of cell surface-associated p75NTR following PMA treatment. C) p75NTR internalization is induced by PMA and NGF. Serum-starved PC12 cells were incubated for 1 hour at 4°C with Mouse Anti-Rat p75 NGF Receptor (extracellular) Antibody (#AN-170), and following a brief wash were either fixed immediately or incubated with DMSO, PMA, and Native mouse NGF 2.5S protein (99%) or Native mouse NGF 2.5S protein (>95%) (#N-240 or #N-100 respectively) prior to fixation. After fixing and blocking, cells were incubated first with donkey anti-mouse Alexa-647 (red) for labeling cell surface associated Mouse Anti-Rat p75 NGF Receptor (extracellular) Antibody and then permeabilized and incubated with donkey anti-mouse Alexa-488 (green) for labeling Mouse Anti-Rat p75 NGF Receptor (extracellular) Antibody, and washed. D) Quantification of p75NTR internalization induced by PMA and NGF.
Adapted from reference 17 with permission of The American Society for Biochemistry and Molecular Biology.
References
- Bronfman, F.C. et al. (2003) J. Neurosci. 23, 3209.
- Butowt, R. and von Bartheld, C.S. (2003) Eur. J. Neurosci. 17, 673.
- Gil, C. et al. (2007) FEBS Lett. 581, 1851.
- Grimes, M.L. et al. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 9909.
- Howe, C.L. et al. (2001) Neuron 32, 801.
- Huovila, A.J. et al. (2005) Trends Biochem. Sci. 30, 413.
- Jung, K. et al. (2003) J. Biol. Chem. 278, 42161.
- Kaasinen, S.K. et al. (2008) Int. J. Devl. Neurosci. 26, 625.
- Kahle, P. and Hertel, C. (1992) J. Biol. Chem. 267, 13917.
- Kuwako, K. et al. (2004) J. Biol. Chem. 279, 1703.
- Kuwako, K. et al. (2005) J. Neurosci. 25, 7090.
- Lee, R. et al. (2001) Science 294, 1945.
- Reynolds, A.J. et al. (2005) Neurochem. Res. 30, 703.
- Salehi, A.H. et al. (2000) Neuron 27, 279.
- Tcherpakov, M. et al. (2002) J. Biol. Chem. 277, 49101.
- Urano, Y. et al. (2005) J. Lipid Res. 46, 904.
- Urra, S. et al. (2007) J. Biol. Chem. 282, 7606.
- Weskamp, G. et al. (2004) J. Biol. Chem. 279, 4241.