It has been more than thirty years since the discovery of a small tetradecapeptide that was able to inhibit the release of growth hormone from the hypothalamus1. The peptide called somatotrophin release inhibiting factor (SRIF) or somatostatin (SST), has been found to be involved in an astounding array of biological effects. SST acts through a family of five G protein-coupled receptors (GPCRs), known as SSTR1 to SSTR5, to carry out its biological activities that include modulation of neurotransmission, inhibition of endocrine secretion, inhibition of cell proliferation and smooth muscle contractility.
Since the cloning and identification of the five SST receptors, an increasing body of information has shed light on the molecular biology, pharmacology and physiological and pathological function of this intriuiging peptide. The recent advances in this field will be briefly summarized below.
Somatostatin Production and Secretion
SST is produced in neurons and secretory cells in the central and peripheral nervous system and in the gastrointestinal tract. However, a number of additional SST synthesis sites have been identified and include the placenta, kidney, retina and cells of the immune system.
In neurons and peripheral secreting cells, SST release is activated by membrane depolarization and/or increasing cytosolic Ca2+ concentration2-4.
SST release can be stimulated by a variety of hormones, neuropeptides, neurotransmitters, cytokines, growth factors and nutrients. For example, growth hormone-releasing hormone (GHRH), neurotensin and corticotropin-releasing hormone (CRH) are all potent stimulators of SST secretion in several tissues. On the other hand, the neurotransmittersγ aminobutiric acid (GABA) and opiates generally inhibit SST secretion. Similarly the inflammatory cytokines IL-1, TNFα and IL-6 stimulate while TGFβ and leptin inhibit SST secretion2.
SST biosynthesis involves the synthesis of a large preproSST precursor molecule that is subsequently proteolyticaly processed to yield two bioactive peptides: the originally discovered 14 amino acid peptide (SST-14) and the C-terminally extended form of 28 amino acids (SST-28). All five SSTRs recognize both cleavage peptides with high affinity while only SSTR5 shows a binding preference for SST-28 over SST-14 (see Table)2-4. Recently, a novel SST-like peptide called cortistatin (CST) that also gives rise to two cleavage products CST-17 and CST-29, has been identified. CST, which takes its name from its predominantly cortical expression, is able to bind all five SSTRs with nanomolar affinities and shares many of the pharmacological and functional activities of SST including depression of neuronal activity. However, CST also has a number of distinct biological activities including slow-wave sleep induction and depression of locomotor activity, thus suggesting the presence of a specific CST receptor5. Indeed, the orphan GPCR receptor MrgX2 has been lately identified as the specific CST receptor6.
The Somatostatin Receptors
As mentioned above, SST acts on its multiple cell targets via a family of six receptors that originate from five genes: SSTR1, SSTR2a, SSTR2b, SSTR3, SSTR4, SSTR5. SSTR2 is alternatively spliced at its C-terminus producing the SSTR2a and the SSTR2b variants that have a somewhat different tissue distribution. The different SSTRs are expressed throughout the central nervous system (CNS) as well as in peripheral tissues like pancreas, stomach, small intestine, etc. (see Table for details).
Like SST itself SSTR expression can be modulated by several factors. First, as in most GPCRs, ligand binding to the receptor induces either receptor internalization and/or uncoupling of the receptor from the G-proteins resulting in receptor desensitization. Second, several hormones such as estrogen and thyroid hormone can regulate SSTRs expression in several tissues at the transcriptional level2.
Besides their expression in normal tissues, SSTRs have been identified in tumor cell lines of different etiology including pituitary, pancreatic, breast and hematopoietic. Moreover, the majority of human tumors do express SSTRs, often more than one receptor subtype. In general, SSTR2 is the most common SSTR subtype found in human tumors followed by SSTR1 with SSTR3 and SSTR4 being less common. SSTR5 appears to be more tumor specific with strong expression in some tumors (i.e. breast) and complete absence in others (i.e. pancreatic)2,7,8. The high frequency of SSTR expression in human tumors has been exploited therapeutically in various ways as described later.
Signal Transduction of Somatostatin Receptors
As mentioned above, the SSTRs are members of the GPCR superfamily and as such modulate cellular function through multiple pathways coupled to G-protein dependent signaling pathways. The different signaling pathways activated by the various SSTR subtypes vary according to the receptor subtype and tissue localization. However, all SSTR subtypes inhibit adenylate cyclase and cAMP production upon ligand binding2-4.
A second signaling pathway that is activated following engagement of all SSTR subtypes (save for SSTR1) is the activation of G-protein regulated inward rectifier (GIRK or Kir3) K+ channel family9. Activation of these K+channels leads to depolarization of the cell membrane and consequently to a decrease in Ca2+ flux through voltage-dependent Ca2+ channels leading to a decrease in intracellular Ca2+ concentration. As reduced cytosolic cAMP levels and intracellular Ca2+ concentrations are known to induce blocking of regulated secretion, activation of these two signaling pathways could explain the inhibitory effects of SST in neurotransmitter and hormone secretion10.
A third pathway linked to SST signaling is the regulation of protein phosphatases. SST activates (upon binding to its receptor) a number of protein phosphatases from different families including serine/threonine phosphatases, tyrosine phosphatase (i.e. SHP-1 and SHP-2) and Ca2+-dependent phosphatases (i.e. calcineurin)11,12.
Somatostatin Function in Health and Disease
Virtually all of the pleiotropic effects of SST in the different target tissues can be explained by two basic biological mechanisms: inhibition of secretion and inhibition of proliferation.
As already mentioned, the SST peptides inhibit secretion (of neurotransmitters or hormones) from cells in different tissues such as the pituitary gland, the endocrine pancreas and the stomach. The molecular mechanism by which SST exerts its inhibitory effects on cell secretion, is still a matter of intense study and may vary between the different cell types. However, it is generally accepted that both the decrease in intracellular cAMP and Ca2+ is mainly responsible for the observed inhibition of secretion, with some effect due to the activation of phosphatases such as calcineurin2,10.
SST has anti-proliferative effects in several normal dividing cells such as intestinal mucosal cells, inflammatory cells and activated lymphocytes. The molecular mechanism involved is less well understood than the one involved in the inhibition of secretion. The anti-proliferative effects of SST are largely believed to be due to the activation of tyrosine phosphatases that dephosphorylate (and inhibit) growth factor receptor such as the epidermal growth factor receptor (EGFR). In addition, the SST-mediated activation of phosphatases regulates more distal signaling pathways such as the MAPK pathway. Addition of SST (or synthetic analogues) to SSTR expressing proliferating cells usually produces cell growth arrest at the G1 phase of the cell cycle13. Interestingly, in some cells, activation of the SSTR2 and SSTR3 subtypes induced apoptosis and cell death rather than growth arrest through activation and upregulation of the tumor suppressor p53 and the pro-apoptotic protein Bax14.
SST and SSTR function has also been involved in several pathological conditions such as Alzheimer’s disease, neuroendocrine dysfunctions and several types of cancer.
In fact, the expression of SSTRs in several human tumors was so pervasive that it helped create an entire new field in oncology: peptide therapy. More than fifteen years ago a synthetic radiolabeled SST analog was used to localize neuroendocrine tumors and its metastasis in vivo by scintigraphy, a technique where binding of the radiolabeled peptide to the SSTR could be detected as hot spots by γ camera scan15. The technique is still considered the most accurate for the diagnosis of cancers of neuroendocrine origin.
SST analogs have also been used in direct tumor reduction with 90Y radiolabeled analogs and in the symptomatic treatment of hormone secreting tumors8,16.

Abbreviations: SSTR: Somatostatin Receptor; SST: Somatostatin; GI tract: Gastrointestinal tract.
Conclusion
Since its discovery more than thirty years ago, much progress has been made in understanding the pathological and physiological function of SST and its receptors. The variety of target tissues and cell pathways involved in the biological functions of SST, promise to keep scientists engaged in this field for many years to come. A better elucidation of the different signaling pathways engaged by the different SSTR subtypes should be attempted, as well as a better understanding of the SSTR subtype tissue distribution in normal and diseased states. For this endeavor highly specific antibodies to both intra- and extracellular epitopes of the different SSTR subtypes will be indispensable. The new insights gained from these studies are eagerly awaited.
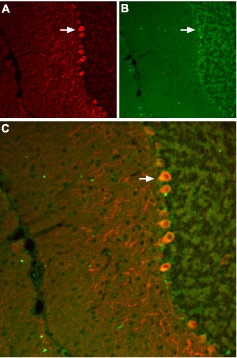
Staining of SSTR3 receptor with Anti-SSTR3 antibody (#ASR-003) in mouse cerebellum. A, SSTR3 appears in Purkinje cells (red, arrow points at an example). B, staining of Purkinje nerve cells with mouse anti parvalbumin (a calcium binding protein, green). C, Confocal merge of SSTR3 and parvalbumin demonstrates the co-localization of these proteins in Purkinje cells (white arrow points at a Purkinje cell pointed at in A and B).

1. Anti-SSTR3 antibody (#ASR-003) (1:200).
2. Anti-SSTR3 antibody, preincubated with the negative control antigen.
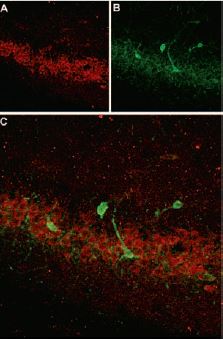
Immunohistochemical staining of somatostatin receptor 4 (SSTR4) with Anti-SSTR4 antibody (#ASR-004) in rat hippocampus. A, SSTR4 appears in the pyramidal layer (red). B, Staining of interneurons with mouse anti parvalbumin (PV, green). C, Confocal merge of SSTR4 and PV demonstrates separate localization in hippocampus.
1. Anti-SSTR4 antibody (#ASR-004) (1:200).
2. Anti-SSTR4 antibody, preincubated with the negative control antigen.

1. Anti-SSTR1 antibody (#ASR-001) (1:200).
2. Anti-SSTR1 antibody, preincubated with the negative control antigen.
References
- Brazeau, P. et al. (1973) Science 179, 77.
- Patel, Y.C. (1999) Front. Neuroendocrinol. 20,157.
- Csaba, Z. and Dournaud, P. (2001) Neuropeptides 35, 1.
- Olias, G. et al. (2004) J. Neurochem. 89, 1057.
- Spier, A.D. and de Lecea, L. (2000) Brain Res. Rev. 33, 228.
- Robas, N. et al. (2003) J. Biol. Chem. 278, 44400.
- Hofland, L.J. and Lamberts, S.W. (2001) Ann. Oncol. 12, S31.
- Reubi, J.C. (2003) Endocr. Rev. 24, 389.
- Kreienkamp, H.J. et al. (1997) FEBS Lett. 419, 92.
- Bousquet, C. et al. (2001) Chemotherapy 47, 30.
- Lopez, F. et al. (1997) J. Biol. Chem. 272, 24448.
- Renstrom, E. et al. (1996) Neuron 17, 513.
- Ferjoux, G. et al. (2000) J. Physiol. Paris 94, 205.
- Sharma, K. and Srikant, C.B. (1998) Int. J. Cancer 76, 259.
- Krenning, E.P. et al. (1989) Lancet 1, 242.
- Kaltsas, G.A. et al. (2005) Endocr. Relat. Cancer 12, 683.