TRP channels show diverse biophysical properties and gating mechanisms and were found to play an important role in sensory physiology, being involved in almost every sensory signal initiation from pain sensation to the five senses.
A Remarkable Multifunctional Superfamily
The TRP superfamily is one of the largest ion channel families and consists of diverse groups of proteins. In mammals, about 28 genes encode the TRP ion channel subunits. The mammalian TRP superfamily comprises six subfamilies known as the TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPML (mucolipins), TRPP (polycystin) and the TRPA (ANKTM1) ion channels1-4. There is a common structural homology unites the TRPs’. All have a putative six-transmembrane domains (TM) with a pore domain between the fifth and the six TM, and all assemble as tetramers. Both the N- and the C-termi of all TRPs are intracellular3. The N-terminal of some TRPs (TRPC, TRPV and TRPA) contain 3-6 ankyrin repeats that mediate cytoskeletal anchoring and apparently protein-protein interactions. The TRPCs and the TRPMs contain TRP domains at their C-terminals adjacent to the transmembrane domain5-6. The amino acid sequences of the entire TRP family are only 20% homologous, mainly in the transmembrane domains, however, within each family there is a high degree of homology along the entire sequence5.
The TRP channels are widespread, and are either specifically or ubiquitously expressed in excitable and non-excitable cells1-6,50. The selectivity of the TRPs varies widely between the different members of the family and with regard to different cations5. TRP channels show diverse biophysical properties and gating mechanisms and were found to play an important role in sensory physiology, being involved in almost every sensory signal initiation from pain sensation, to the five senses. It also plays a major role in transepithelial Ca2+ and Mg2+transport.
The Ca2+ Entry Channels
The TRPC, TRPV and TRPP Subfamilies
Many cellular processes such as gene expression, secretion, proliferation and apoptosis, are regulated by Ca2+ which is considered to be one of the most ubiquitous second messengers and an important cofactor for the function of many proteins7,8. Thus, from yeast to mammals, different mechanisms that tightly regulate Ca2+homeostasis have been evolved including membrane channels and pumps7,8. In excitable cells, voltage gated Ca2+ channels and ligand gated channels regulate Ca2+ entry8. However, in non-excitable cells, Ca2+ entry is mostly regulated by non-voltage gated channels such as receptor-operated channels (ROCs) or store operated channels (SOCs)3,8,9. Accumulating data suggests the involvement of TRPC channels and members of the TRPV and TRPP subfamilies in one or both mechanisms.
The TRPC Subfamily
The TRPC subfamily consists of seven proteins named TRPC1 to 7 which can be further divided into four subgroups based on their sequence homology and functional similarities: (1) the TRPC1 (2) TRPC4 and TRPC5 (3) TRPC3, TRPC6 and TRPC7 (4) TRPC22,10. They are highly expressed in the central nervous system and to a lesser extent in peripheral tissues. TRPC1 was the first mammalian TRP protein that was reported to form an ion channel2,11. It can co-assemble with other TRPC subunits (TRPC3, TRPC4, TRPC5) to form heterotetramers whose properties are distinct from that of their homomeric form. The existence of the TRPC1 homomers has not been established as yet1-3. TRPC6 can form heterotetramers with TRPC3 and TRPC7. It is primarily expressed in brain, lung and muscle. High levels of expression of the channel were also found in human platelets. Recently it was reported that TRPC6 is also expressed in the kidney where a mutated channel has been implicated in kidney failure disease12,13.
Activation of TRPC Channels
All members of the TRPC subfamily are involved in Ca2+ homeostasis. In general, TRPCs are Ca2+/cation selective channels that are activated by Ca2+ store depletion, diacylglycerol (DAG), or Inositol-1,4,5-Trisphosphate (IP3)14. The function and regulation of the TRPC Ion channels remain elusive due to conflicting data and interpretations made by different investigators15. As was mentioned above, while trying to solve the mystery of the TRPC gating mechanism two main mechanisms were proposed and explored:
A. The receptor operated mechanism (ROCs) has been described as “the most likely mechanism for TRPC channels…” on the basis that all mammalian TRPC channels can be regulated as a result of G-protein coupled receptor (GPCRs) activation3. Activation of GPCRs such as muscarinic type 1, regulates the activity ofTRPC1 and TRPC4, while histaminergic type 1 activates heteromers of TRPC3 and TRPC6. Purinergic receptors can activate TRPC73.
B. The store-operated channels (SOCs) which are activated primarily in response to depletion of internal Ca2+stores and can be activated by agonists, intracellular increase in IP3 and by Thapsigargin9,18,19. The mechanism underlying the store operated Ca2+entry is still unresolved.
The finding that some TRPCs might be SOCs while others might be activated by GPCRs or DAG, indicate that TRPC channels can be gated through distinct mechanisms10,19. The TRPC1, TRPC4 and TRPC5 can be activated either by Ca2+ store depletion or by GPCR stimulation pathways, while TRPC3, TRPC6 and TRPC7 form non-selective cationic channels that are activated by the stimulation of GPCRs. TRPC1, TRPC4 and TRPC5 are assumed to form components of store operated channels in some cell types such as salivary gland cells, endothelial cells and vascular smooth muscle cells18. TRPC2 appears to be a pseudogene in humans. However, in other mammals it was located in the rat vomeronasal organ where it is activated through a rise in DAG, and at the head of the sperm in mouse, where it is activated by Ca2+ release, playing an important role in keeping Ca2+ influx after the interaction of the sperm with the egg20,19. Different modes of activation, DAG and SOC, were also described for the TRPC3 channel by different investigators working with heterologous expression systems21,22. Although the accumulating data indicates that TRPCs might function as store operated channels, this role is still very controversial since there is no direct evidence supporting it3. There are claims arguing against the concept that TRPCs may act as store operated channels suggesting that their contribution to changes in Ca2+ influx is the same as all other Ca2+ permeant channels, thus affecting the process of store operated Ca2+ entry indirectly3.
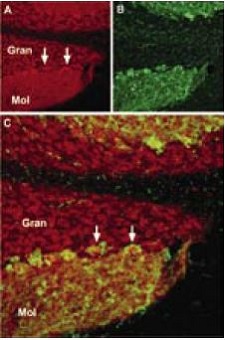
Immunohistochemical staining of TRPC1 with Anti-TRPC1 Antibody (#ACC-010) in mouse cerebellum. (A) TRPC1 (red) appears in Purkinje cells (arrows) and in the molecular (Mol) and granule (Gran) layers. (B) staining with mouse anti parvalbumin in the same brain section. (C) Confocal merge of TRPC1 and PV demonstrates partial co-localization in the Purkinje and the molecular layers.
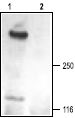
1. Anti-TRPC1 Antibody (#ACC-010) (1:200).
2. Anti-TRPC1 Antibody, preincubated with the negative control antigen.
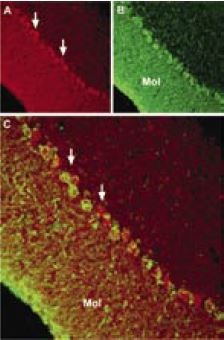
Immunohistochemical staining of transient potential channel 6 (TRPC6) with Anti-TRPC6 Antibody (#ACC-017) in mouse cerebellum. A, TRPC6 (red) appears in Purkinje cells (arrows) and in the molecular (Mol) layer. B, staining with mouse anti parvalbumin in the same brain section. C, Confocal merge of TRPC6 and PV demonstrates partial co-localization in the Purkinje and the molecular layers.

Immunohistochemical staining of TRPC4 with Anti-TRPC4 Antibody (#ACC-018) in mouse cerebellum. (A) TRPC4 (red) appears in Purkinje cells (arrows) and in the molecular (Mol) layer. (B) staining with mouse anti parvalbumin in the same brain section. (C) Confocal merge of TRPC4 and PV demonstrates partial co-localization in the Purkinje and the molecular layers.
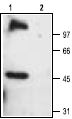
1. Anti-TRPC6 Antibody (#ACC-017) (1:200).
2. Anti-TRPC6 Antibody, preincubated with the negative control antigen.
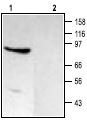
1. Anti-TRPC4 Antibody (#ACC-018) (1:200).
2. Anti-TRPC4 Antibody, preincubated with the negative control antigen.

Jurkat cells were grown to 70% confluency. Then 1 μM Thapsigargin (#T-650) or vehicle were added for 6 hours. At the end of the incubation period, the cells extracts were detected for cleaved Caspase 3 with Caspase 3 specific antibodies.
The Epithelial Ca2+ Channels
The TRPV5, TRPV6 and the TRPP1
In addition to the TRPC, other members of the TRP superfamily are also considered to be Ca2+ entry channels. TRPP2 (PKD2) and two members of the TRPV subfamily (see below), TRPV5 (also known as ECaC1), and TRPV6 (CaT1) function as epithelial Ca2+ channels (TRPV in the kidney and TRPV6 in the intestine). TRPV5 and TRPV6 form constitutively open channels, which are highly Ca2+ selective23,24. TRPV5 is preferentially expressed in the kidney whereas TRPV6 is more prominently expressed in the intestine. However, expression of both channels has also been detected in placenta, prostate, pancreas, salivary gland and colon. The expression of both channels and Ca2+ absorption by them is regulated by vitamin D3 metabolites. An increase in dietary Ca2+ induces an increase in vitamin D3 resulting in elevated expression of TRPV5 and TRPV6 mRNA25,26.However, it is likely that Ca2+ responsive elements, such as serum-responsive element and cAMP/Ca2+-responsive element might be also involved27.
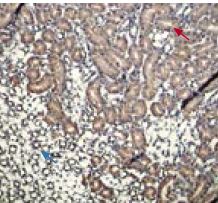
Immunohistochemical staining of TRPV5 with Anti-TRPV5 Antibody (#ACC-035) in rat kidney. Section of the outer and inner stripe of outer medulla. Positive staining of the thick ascending limb of the Henle’s loop in inner stripe (blue arrow) and of the pars recta of the proximal tubule in the outer stripe (red arrow). DAB product is brown and counterstain is Cresyl Violet.
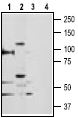
1,2. Anti-TRPV5 Antibody (#ACC-035) (1:200)
3,4. Anti-TRPV5 Antibody, preincubated with the negative control antigen.
The Sensing TRP Channels
The TRPV, TRPM, and the TRPA Subfamilies
Thermosensation
Six-thermosensitive ion channels have been described, all members of the TRP family (thermoTRPs). Each channel exhibits distinct thermal activation thresholds ranging from noxious cold (≤ 17°C) to noxious heat (≥ 52°C)29,30.
The hot TRPs: The TRPV subfamily consists of six members named TRPV1-6. Four of them (TRPV1 to TRPV4) are thermosensation ion channels. The most established member of this family is the TRPV1 (previously also known as the capsaicin receptor or vanilloid receptor, VR1). Its involvement in thermal nociception has been well documented by different methods29.TRPV1 is expressed predominantly in nociceptors and in sensory neurons and is activated by moderate heat (≥43°C) and by capsaicine16,29,31. xtracellular ATP was shown to potentiate TRPV1 through the P2Y1 receptor in both heterologous systems and rat DRG.16 Pain behavior and nociceptors activity that is induced by capsaicin (TRPV1 activator) are reduced once Somatostatin receptors (SSTRs) are activated by their agonists17.
The TRPV2 (VRL-1) however, has a higher threshold for activation by heat (≥52°C). It shares a 50% homology with TRPV1 but is not activated by capsaicin or by low pH32. Both TRPV3 and TRPV4 are activated at a lower temperature threshold above 31°C and 25°C respectively. Both channels are expressed in DRG. However, TRPV3 is also expressed in mouse keratinocytes but not in mouse DRG, while TRPV4 is expressed in a wide variety of tissues and may also have a possible role as mechanosensitive channel33.
The cold TRPs: TRPM8 and TRPA1 are considered to be the cold ion detection channels. The TRPM subfamily consists of eight members, TRPM1 to TRPM8, which also can be further subdivided into four subgroups based on their sequence homology:
- TRPM1 and TRPM3
- TRPM6 and TRPM7
- TRPM4 and TRPM5
- TRPM2 and TRPM834.
TRPM8 is activated at a temperature threshold below 28°C and also by menthol and other cooling agents such as icilin35. The channel activation by cold or by cooling agents appears to be mediated by different mechanisms35. Expression of TRPM8 was also detected in prostate cancer cells36. The TRPA1 channel (formerly named ANKTM1) is activated by noxious cold temperature (≤17°C) and also by a variety of natural compounds-oils such as cinnamon oil, mustard oil, ginger etc. TRPA1 is expressed in nociceptive neurons expressing TRPV1 as well and might serve as a marker for poly-modal nociceptors37. Three possible mechanisms for the temperature-dependent gating were suggested:
- Production and binding of channel-activating ligand due to temperature changes.
- Temperature dependent rearrangement of the channel structure that leads to channel opening.
- Rearrangement of the lipid bilayer due to temperature dependent changes in membrane tension30.
However, direct evidence to support any of these mechanisms has not yet been found.
Mechanosensation
The human body is constantly bombarded by external mechanical stimuli. The detection and transduction of mechanical stimuli by mechanosensitive channels is crucial in order to maintain normal physiological processes such as balance, touch, hearing and pain38,39. Recent studies have suggested that the TRP channels are mechanosensitive33,40-43. TRPC1 was shown to form the stretch-activated cation channel in vertebrate cells42,43. TRPA1 was suggested to be a mechanosensitive channel in vertebrate hair cells40,42. Activation of TRPV4 homomers by osmotic stress in cultured cells suggested that TRPV4 is also a mechanosensitive channel33. TRPPs form complexes that act as mechanosensory transducers in a variety of biological functions44. They are located at the renal cilia which recently was shown to have sensory function44. Several optional mechanisms have been postulated: activation by membrane lipid tension (TRPC1), direct activation by mechanical force delivered through structural proteins (TRPA, TRPP) and activation by second messenger (TRPV4).
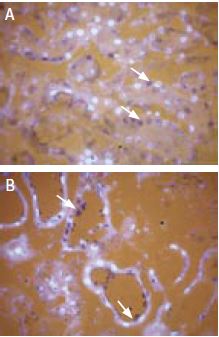
Immunohistochemical staining of TRPV1 in kidney using Anti-TRPV1 (VR1) Antibody (#ACC-030). Selective subsets of cells were stained in mouse (A) compared to rat (B).
Diaminobenzidine color product is blue-black (arrows). The fluorescent counterstain is DAPI (silver).
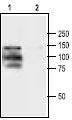
1. Anti-TRPV1 (VR1) Antibody (#ACC-030) (1:200).
2. Anti-TRPV1 (VR1) Antibody, preincubated with the negative control antigen.
In Conclusion
The TRPs belong to a remarkably multifunctional and complex superfamily. TRP ion channels participate in almost all known sensory responses and sometimes in more than one at a time. TRPC1 and TRPA1 are Ca2+entry channels and mechanosensitive channels. TRPV4 was found to be a thermo and mechanosensative channel. TRP channels were shown to play an important role in complex mechanisms such as pulmonary artery myocyte cell proliferation45,46 and in chemotropic axon guidance47-49. The large number of TRP channels, the diversity of functions and expression pattern along with poorly understood mechanisms of activation make it possible that more novel functional properties of TRP members might be discovered3,6.
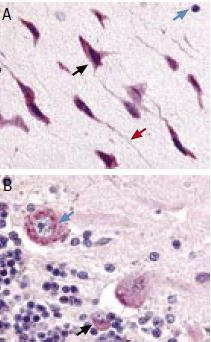
Immunohistochemical staining of TRPC5 with Anti-TRPC5 Antibody(#ACC-020) in rat brain cortex and cerebellum. (A) Picture showing the third layer of the brain cortex. Pyramidal neurons cells (black arrow) and their axons (red arrow) have shown strong staining. However, glial cells (blue arrows) show no staining at all. (B) Picture showing the Purkinje layer of the rat cerebellum. Note that Purkinje cells (blue arrows) and Gologi II Cells (black arrows) were strongly stained. Staining product is red and counterstain is hematoxylin.
Immunohistochemistry data provided by LifeSpan Biosciences, Seattle, USA.

Immunohistochemical staining of TRPV4 channel with Anti-TRPV4 Antibody (#ACC-034) in rat dorsal root ganglion (DRG). (A) TRPV4 channel (red) in DRG neurons. (B) staining with mouse anti Parvalbumin (green) in the same DRG section. (C) Confocal merge of TRPV4 and Parvalbumin demonstrates existence of the parvalbumin calcium binding protein in a subset of neurons expressing TRPV4.

1. Anti-TRPC5 Antibody (#ACC-020) (1:200)
2. Anti-TRPC5 Antibody, preincubated with the negative control antigen.

1. Anti-TRPV4 Antibody (#ACC-034) (1:200).
2. Anti-TRPV4 Antibody, preincubated with the negative control antigen.
References
- Moran, M.M. et al. (2004) Current Opin. Neurobiol. 14, 362.
- Clapham, D.E. et al. (2003) Pharmacol. Rev. 55, 591.
- Clapham, D.E. (2003) Nature 426, 517.
- Padinjat, R. and Andrews, S. (2004) J. Cell. Sci. 117, 5707.
- Huang, C.L. (2004) J. Am. Soc. Nephrol. 15, 1690.
- Voets, T. and Nilius, B. (2003) J. Membrane Biol. 192, 1.
- Bonilla, M. and Cunningham, K.M. (2002) Sci. STKE. 127, PE17.
- Wu, X. et al. (2004) J. Biol. Chem. 279, 43392.
- Liu, X et al. (2005) J. Biol. Chem. in press.
- Montell, C. (2001) Sci. STKE. 90, re1.
- Zitt, C. et al. (1996) Neuron 16, 1189.
- Winn. M.P. et al. (2005) Science 308, 1801.
- Reiser, J. et al. (2005) Nat. Genet. 37, 739.
- Mauro, T. (2003) J. Invest. Dermatol. 121, IX.
- Putney, J.W.Jr. (2004) Trends in Cell Biology 14, 282.
- Tominaga, M. et al. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6951.
- Carlton, S.M. et al. (2004) Pain 110, 616.
- Liu, X. et al. (2003) J. Biol. Chem. 278, 11337.
- Montell, C. (2005) Sci. STKE. 2005, re3.
- Vennekens, R. et al. (2002) Cell Calcium 31, 253.
- Vazquez, G. et al. (2003) J. Biol. Chem. 278, 21649.
- Venkatachalam, K. et al. (2003) J. Biol. Chem. 278, 29031.
- Bodding, M. and Flockerzi, V. (2004) J. Biol. Chem. 279, 36546.
- Peng, J.B. et al. (2003) J. Physiol. 551, 729.
- Hoenderop, J.G.J. et al. (2005) Physiol. Rev. 85, 373.
- Van de Graaf. S.F.J. et al. (2004) J. Steroid. Biochem. Mol. Biol. 89-90, 303.
- Hoenderop, J.G.J. et al. (2003) Pflugers Arch- Eur. J. Physiol. 446, 304.
- Guler, A.D. et al. (2002) J. Neurosci. 22, 6408.
- Tominaga, M. and Caterina, M.J. (2004) J. Neurobiol. 61, 3.
- Voets, T. (2004) Nature 430, 748.
- Sugiura, T. et al. (2002) J. Neurophysiol. 88, 544.
- Muraki, K. et al. (2003) Circ. Res. 93, 829.
- Corey, D.P. (2003) Neuron 39, 585.
- Harteneck, C. (2005) Naunyn Schmiedebergs Arch Pharmacol. 371, 307.
- McKemy, D.D. (2005) Molecular Pain 1, 16.
- Clapham, D.E. (2002) Science 295, 2228.
- Bandell, M. et al. (2004) Neuron 41, 849.
- Lewin, G.R. and Moshourab, R. (2004) J. Neurobiol. 61, 30.
- Jerman, A.P. (2002) Human Molecular Genetics 11, 1215.
- Corey, P.D. et al. (2004) Nature 432, 723.
- Lin, S.Y. and Corey, P.D. (2005) Curr. Opin. Neurobiol. 15, 1.
- Barritt, G. and Rychkov, G. (2005) Nature Cell Biology. 7, 105.
- Maroto, R. et al. (2005) Nature Cell Biology 7, 179.
- Delmas, P. (2004) Cell 118, 145.
- Landsberg, J.W. and Yuan, J.X. (2004) News Physiol. Sci. 19, 44.
- Golovina, V.A. et al. (2001) Am. J. Physiol. Heart Circ. Physiol. 280, H746.
- Wang, G.X. and Poo, M. (2005) Nature 434, 898.
- Li, Y. et al. (2005) Nature 434, 894.
- Gomez, T. (2005) Nature 434, 835.
- Minke, B. and Cook, B. (2002) Physiol. Rev. 82, 429.