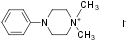Overview
- Bertrand, D. et al. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 1993.
 Alomone Labs 1,1-Dimethyl-4-phenylpiperazinium iodide activates muscle fetal α1/β1/γ/δ nAChR heterologously expressed in Xenopus oocytes.A. Time course of current repetitive and reversible activation by 100 µM 1,1-Dimethyl-4-phenylpiperazinium iodide (#D-125). Holding potential was -60 mV and currents were elicited by application of 100 µM acetylcholine. B. Example traces of current responses to 10 mM or 100 mM acetylcholine as indicated.
Alomone Labs 1,1-Dimethyl-4-phenylpiperazinium iodide activates muscle fetal α1/β1/γ/δ nAChR heterologously expressed in Xenopus oocytes.A. Time course of current repetitive and reversible activation by 100 µM 1,1-Dimethyl-4-phenylpiperazinium iodide (#D-125). Holding potential was -60 mV and currents were elicited by application of 100 µM acetylcholine. B. Example traces of current responses to 10 mM or 100 mM acetylcholine as indicated.
- Blanchet, M.R. et al. (2007) J. Leuk. Biol. 81, 1245.
- Dorion, G. et al. (2005) Am. J. Physiol. 288, L1139.
- Guandalini, L. et al. (2005) Farmaco 60, 99.
- Bertrand, D. et al. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 1993.
- Kalra, R. et al. (2004) Clin. Diagn. Lab. Immunol. 11, 563.
- Matsunaga K. et al. (2001) J. Immunol. 167, 6518.
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels comprising five subunits. The muscle-type receptor is made of two α1, β, γ and δ subunits, while the neuronal nicotinic receptors are formed by different combinations of two α and three β subunits. These receptors are expressed on neuronal and different types of non-neuronal cells such as, lymphocytes, alveolar macrophages, airway smooth muscle cells, epithelial cells, and fibroblasts1.
nAChRs mediate numerous specific physiological functions depending on the type of cell involved. For example, nicotine induces the release of dopamine in the central nervous system and stimulates nitric oxide (NO) synthase gene expression in endothelial cells2.
1,1-Dimethyl-4-phenylpiperazinium iodide (DMPP) is a selective nAChR agonist. For a long time, DMPP was considered to be the prototype of ganglionic stimulating agent, since it was able to activate the nicotinic receptor in sympathetic ganglia with higher potency than the muscle-type. However, it has since been shown that DMPP binds with higher affinity to the nicotinic receptor in the brain3. DMPP activates α4 nicotinic channels expressed in Xenopus oocytes with IC50 of 0.77 uM4.
Nicotinic agonists, such as DMPP, have anti-inflammatory effects in mouse models of hypersensitivity pneumonitis, asthma, airway inflammation, and Type I diabetes5. DMPP reduces the release of IL-1 and TNF from isolated spleen cells, and the release of TNF and IL-6 from macrophages6.
1,1-Dimethyl-4-phenylpiperazinium iodide (#D-125) is a highly pure, synthetic, and biologically active compound.
Applications
Citations
- Burton, S.D. et al. (2017) J. Neurosci. 37, 1117.