Overview
- Peptide (C)RQSVELHSPQSLPRGSKA, corresponding to amino acid residues 254-271 of rat AQP2 (Accession number P34080). Intracellular, C-terminus.
- Rat kidney sections. Mouse paraffin-embedded kidney sections (1:200) (Kim, J.I. et al. (2013) Am. J. Physiol. 304, F1283.).
Aquaporin 2 (AQP2) belongs to a family of membrane proteins that allow passage of water and certain other solutes through biological membranes. The family is composed of 13 members (AQP0 to AQP12). Little is known about the function of the two newest members, AQP11 and AQP12.
The aquaporins can be divided into two functional groups based on their permability characteristics: the aquaporins that are only permeated by water and the aquaglyceroporins that are permeated by water and other small solutes such as glycerol. AQP2 together with AQP1, AQP4 and AQP5 belongs to the first group.1
The proteins present a conserved structure of six transmembrane domains with intracellular N- and C-termini. The functional channel is a tetramer but each subunit has a separate pore and therefore the functional channel unit, contains four pores.1
AQP2 expression is largely confined to the kidney, particularly in the renal collecting duct where it performs a key role in water absorption and urine concentration. In fact, mutations in the AQP2 gene produce hereditary nephrogenic diabetes insipidus, a disorder that results in the excretion of large volumes of urine.2
Under normal conditions, water homeostasis in the kidney is regulated through the anti-diuretic hormone vasopressin. Vasopressin is secreted from the pituitary gland and transported to the kidney through the blood where it binds to its receptor that is mainly expressed in cells of the collecting duct. The activated vasopressin receptor induces an increase in intracellular cAMP and subsequent PKA activation, which phosphorylates AQP2. This phosphorylation causes the translocation of AQP2 channels from intracellular vesicles to the cell membrane where it markedly increases water permeability.1,3,4
Application key:
Species reactivity key:
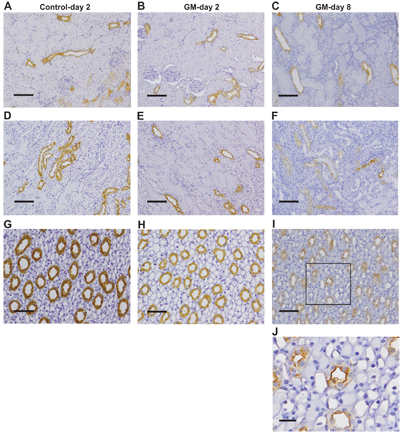 Expression of AQP2 in rat kidney sections before and after gentamicin treatment.Immunohistochemical staining of rat kidney sections using Anti-Aquaporin 2 Antibody (#AQP-002) after GM treatment. Kidney sections from rats after 2 days of treatment with vehicle (control; A, D, and G) or GM (B, E, and H) or 8 days of treatment with GM (C, F, I, and J). Representative images of the cortex (A–C), outer medulla (D–F), and inner medulla (G–J) are shown. The box in I indicates the region of high magnification in J. Brown staining indicates the presence of AQP2.Adapted from Abdeen, A. et al. (2014) with permission of the American Physiological Society.
Expression of AQP2 in rat kidney sections before and after gentamicin treatment.Immunohistochemical staining of rat kidney sections using Anti-Aquaporin 2 Antibody (#AQP-002) after GM treatment. Kidney sections from rats after 2 days of treatment with vehicle (control; A, D, and G) or GM (B, E, and H) or 8 days of treatment with GM (C, F, I, and J). Representative images of the cortex (A–C), outer medulla (D–F), and inner medulla (G–J) are shown. The box in I indicates the region of high magnification in J. Brown staining indicates the presence of AQP2.Adapted from Abdeen, A. et al. (2014) with permission of the American Physiological Society.
