Blocking Peptide Protocol for Western Blot (WB)
Ensure primary antibody specificity in western blotting with a simple blocking peptide control.
A blocking peptide is the original antigen we use for immunization during antibody generation. This makes blocking peptides good controls to help validate antibody specificity. Sometimes called “immunizing peptides” or “negative control antigens,” a blocking peptide works as a control by competing with, or blocking, the primary antibody.
You can easily use a blocking peptide control alongside your western blot (WB) to show that your antibody binds the target it is supposed to. It’s important to remember that a blocking peptide is just one of the tools you should use when setting up proper controls for your WB.
An antibody blocked with a blocking peptide should produce no signal when added to your membrane. Any positive results with the blocking peptide control means the antibody is binding to a protein besides the intended target.
Method
- Reconstitute your lyophilized blocking peptide in 100 µl sterile phosphate-buffered saline (PBS) or double-distilled water (DDW) according to the instructions in the datasheet.
- Optimize your WB protocol. Refer to our WB protocol for more information. Follow the product guidelines or previous experimental optimizations for a recommended dilution for your primary antibody. Determine the quantity of antibody required for two experiments.
- If your optimal antibody concentration is a 1:200 dilution, add 20 µg of antibody to a 1.5 ml Eppendorf tube containing 500 µl of PBS with 1% bovine serum albumin (BSA). Label the tube “antibody alone”.
- To a second identical tube, add 20 µg of antibody and 20 µg of blocking peptide to 500 µl of 1% BSA in PBS. Label the tube “+peptide”.
Note: we recommend beginning with a 1:1 ratio between the antibody and the blocking peptide. You will need to test a series of dilutions to obtain full inhibition.
- Rotate both tubes for 1 hour at room temperature.
- Transfer the contents of each Eppendorf tube to larger tubes and add 4.5 ml of PBS with 1% BSA, 0.1% Tween-20, and 0.05% NaN3 to each tube to get a final antibody dilution of 1:200.
- Add the contents of each tube to its respective membrane test strip for parallel experiments.
- Incubate both membrane strips for 2–3 hours at room temperature or overnight at 4°C with gentle agitation.
- Proceed with the WB protocol, ensuring that you handle both the unblocked and blocked samples in the same way.
- Develop your blots and compare the signal obtained in the two test strips. The band that disappears when using the blocking peptide is specifically recognized by the antibody. Other visible bands represent non-specific antibody binding.
Published customer data using
Alomone Labs blocking peptide controls
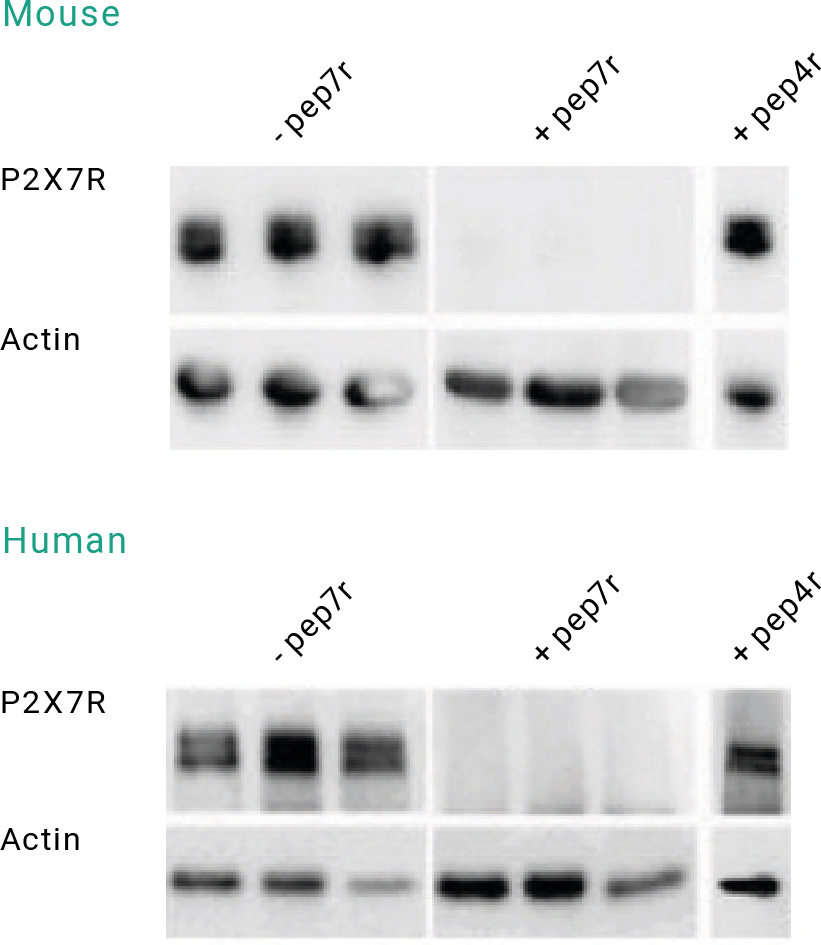
WB using the Anti-P2X7 Receptor Antibody (APR-004) in the presence or absence of blocking peptides.
WBs show a P2X7 receptor band (left panels) in epileptic mouse and human hippocampal samples blotted in the absence of the P2X7 Receptor Blocking Peptide (BLP-PR004) (-pep7r). This band was eliminated (middle panels) when the Anti-P2X7 Receptor Antibody (APR-004) was preincubated with the P2X7 Receptor Blocking Peptide (#BLP-PR004) (+pep7r). In a separate experiment, the anti-P2X7 receptor antibody was preincubated with the P2X4 Receptor Blocking Peptide (BLP-PR002) (+pep4r; right panels). As expected, the P2X4 receptor blocking peptide did not block the anti-P2X7 receptor antibody. The blots were also probed with an antibody directed against actin, which functioned as a loading control.