Western Blot (WB) Protocol for High Molecular Weight (HMW) Proteins
Easily run your western blot with large proteins like membrane channels.
Western blotting high molecular weight (HMW) proteins, like sodium and calcium channels, can be challenging.
Here, we take you through the steps to successfully transfer HMW proteins from your gel to the membrane, all the way through to detection.
If you have any problems, please see our extensive troubleshooting guides.
Sample Denaturation
- Heat the samples in Laemmli buffer at 70–100°C for 10 minutes.
- Load the samples into the gel*.
*Load 80–100 µg tissue lysate/lane or lysate from 2–5 x 105 cells/lane.
| Laemmli Buffer x2 (50 ml) | ||
|---|---|---|
| Reagent | Volume/weight | % of final volume |
| Tris-HCL (0.5 M, PH=6.8) | 12.5 ml | 25% |
| SDS | 10 ml from 20%SDS | 4% |
| Glycerol | 10 ml | 20% |
| 2-Mercaptoethanol | 5 µl | 0.01% |
| Bromphenol Blue sodium salt | 8 mg | 16% |
| DDW | 17.49 ml | 34.98% |
SDS-PAGE
- Run the gel according to the manufacturer’s instructions.
Note: for HMW proteins, Tris-Glycine 4–6% or Tris-Acetate 3–8% gels are the best options, but the gels should be chosen empirically.
Wet Transfer
- Transfer the proteins from the gel to the membrane at 100 mA for 20 hours at 4°C.
Note: this step is crucial since it determines the effectiveness of the transfer. A longer transfer time will increase the efficiency of HMW protein transfer.
Western Blotting for HMW proteins
- Block the membrane with Blocking Solution (PBS with 3% BSA and 0.05% NaN3) for 2–5 hours at room temperature with gentle agitation.
- Add primary antibody diluted in PBS with 1% BSA, 0.1% Tween-20, and 0.05% NaN3. Incubate overnight at 4°C with gentle agitation.
Note: if you use a blocking peptide as a negative control, refer to our Peptide Blocking Protocol for WB. - Wash the membrane with Washing Buffer for 3 x 10 minutes at room temperature.
Note: if you are using a secondary antibody conjugated to HRP, do not use solutions containing NaN3 from this point on. - Incubate the secondary antibody in PBS with 1% BSA, and 0.1% Tween-20, for 1 hour at room temperature with gentle agitation.
- Wash the membrane with Washing Buffer for 3 x 10 minutes at room temperature.
- Proceed to detection using an enhanced chemiluminescence (ECL) system.
Note: if using a commercial kit, follow the manufacturer’s instructions. - The membrane exposure time to the film/imager will depend on the abundance of the protein and the detection system.
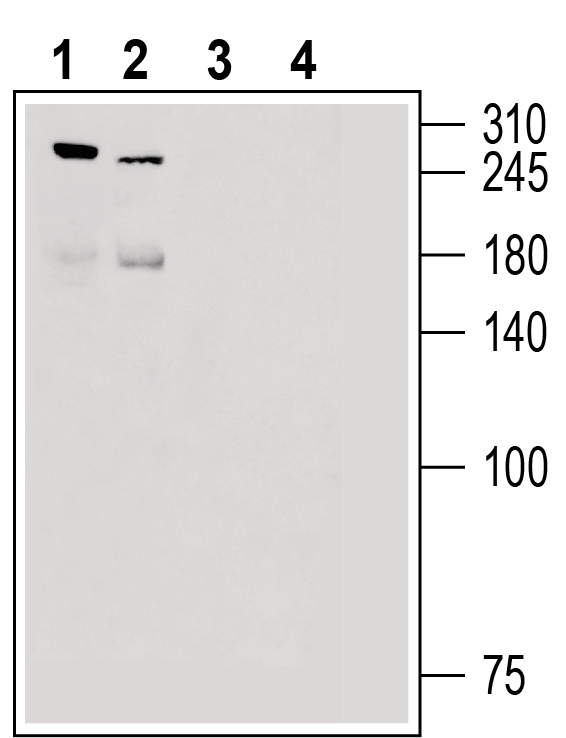
Example Data
Western blot analysis of rat dorsal root ganglion lysate (lanes 1 and 3) and mouse lung lysate (lanes 2 and 4):
1, 2: Anti-Piezo2 Antibody (#APC-090), (1:200).
3, 4: Anti-Piezo2 Antibody, preincubated with Piezo2 Blocking Peptide (#BLP-PC090).