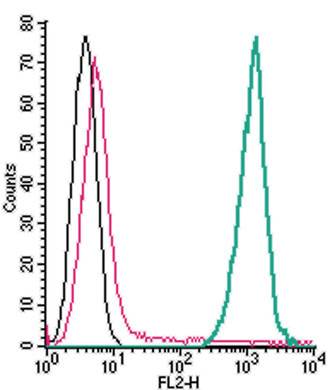Flow Cytometry Protocols for Live Cells: Indirect and Direct Methods
Detailed steps to take you through cell preparation and on to indirect and direct methods of flow cytometry.
Flow cytometry allows you to detect molecules on the cell surface by targeting them with specific antibodies. You can use this to characterize and define cell populations or measure various properties you’re interested in. Indirect flow cytometry used a secondary antibody, conjugated to a reporter, to bind the primary antibody. Direct flow cytometry uses a primary antibody directly conjugated to a reporter.
If you have any problems, please see our extensive troubleshooting guides.
Indirect Flow Cytometry
Cell Preparation
- Transfer 1 x 106 cells into a microtube. Centrifuge for 5 minutes at 200 x g at 4°C. Discard the supernatant.
- Wash the cell pellet by adding 500 µl of ice-cold Labeling Buffer (PBS, 2% BSA or inactivated 3% Fetal Bovine Serum (FBS) and 0.05% NaN3) and resuspend the cell pellet by gentle pipetting. Next, centrifuge for 5 min at 200 x g at 4°C. Discard the supernatant. Repeat twice.
Note: Choosing between BSA and FBS in the labeling buffer depends on the cells type. Inactivate the FBS by heating at 56°C for 30 minutes. - Next, centrifuge for 5 min at 200 x g at 4°C.
- Discard the supernatant.
- Perform steps 2–3 twice in total.
Labeling
- Add primary antibody at the appropriate dilution in 50 µl of Labeling Buffer for each tube.
- Add the primary antibody solution to the cells and resuspend the pellet.
- Incubate on ice for 1 hour.
- Wash away the unbound antibody by adding 500 µl of Labeling Buffer and resuspend the cells by gentle pipetting. Next, centrifuge for 5 minutes at 200 x g at 4°C. Discard the supernatant. Repeat twice.
- Add fluorescently conjugated secondary antibody at the appropriate dilution in 50 µl of ice-cold Labeling Buffer for each tube.
- Incubate on ice for 1 hour, protected from light.
- Wash away the unbound antibody by adding 500 µl of Labeling Buffer and resuspend the cells by gentle pipetting. Next, centrifuge for 5 minutes at 200 x g at 4°C. Discard the supernatant. Repeat twice.
- Resuspend the cells in 1 ml of ice-cold Labeling Buffer. Filter the cell solution into FACS tubes and analyze by flow cytometry.
Direct Live Cell Flow Cytometry Using FITC/PE/APC-Conjugated Primary Antibodies
Cell preparation
- Transfer 1 x 106 cells into a microtube. Centrifuge for 5 minutes at 200 x g at 4°C. Discard the supernatant.
- Wash the cell pellet by adding 500 µl of ice-cold Labeling Buffer (PBS, 2% BSA or inactivated 3% Fetal Bovine Serum (FBS) and 0.05% NaN3) and resuspend the cell pellet by gentle pipetting. Next, centrifuge for 5 min at 200 x g at 4°C. Discard the supernatant. Repeat twice.
Note: Choosing between BSA and FBS in the labeling buffer depends on the cells type. Inactivate the FBS by heating at 56°C for 30 minutes.
Blocking
- Add 50 µl of filtered 2% inactivated normal rabbit serum in PBS and pipette gently.
- Incubate on ice for 10 minutes.
Note: inactivate the NRS by heating at 56°C for 30 minutes.
Labeling
- Add FITC, PE, or APC-conjugated primary antibody at the appropriate dilution in 50 µl of Labeling Buffer for each tube.
- Add the antibody solution to the cells and resuspend the cells.
- Incubate on ice for 1 hour, protected from light.
- Wash away the unbound antibody by adding 500 µl of Labeling Buffer and resuspend the cells by gentle pipetting. Next, centrifuge for 5 minutes at 200 x g at 4°C. Discard the supernatant. Repeat twice.
- Resuspend the cells in 1 ml of ice-cold Labeling Buffer. Filter the cell solution into FACS tubes and analyze by flow cytometry.
Example Data
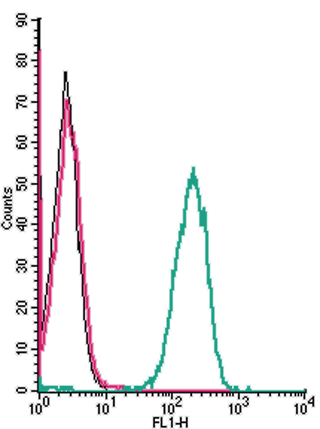
Figure 1. Cell surface detection of Tetraspanin-3 by indirect flow cytometry in live intact human THP-1 monocytic leukemia cell line:
___ Cells.
___ Cells + goat-anti-rabbit-FITC.
___ Cells + Anti-Tetraspanin-3 (extracellular) Antibody (ANR-185), (2.5μg) + goat-anti-rabbit-FITC.
Figure 2. Cell surface detection of TREM2 by direct flow cytometry in live intact mouse J774 macrophage cells:
___ Cells.
___ Cells + Rabbit IgG isotype control-PE.
___ Cells + Anti-TREM2 (extracellular)-PE Antibody (ANR-018-PE), (2.5µg).