Immunohistochemistry (IHC) Protocol for Frozen Heart Sections
Clear and detailed IHC steps for working with heart sections, from tissue preparation to staining.
Immunohistochemistry (IHC) for fresh frozen mouse heart sections requires some adjustments compared to working with other tissues. Here you can find the protocol for this where Immunodetection is by fluorescent microscopy.
If you have any problems, please see our extensive troubleshooting guides.
Tissue preparation
- Remove heart from euthanized mouse.
- Blot out blood and wrap heart in aluminium foil.
- Freeze organ by dipping in liquid nitrogen or by placing on dry ice.
- Store the heart in a 1.5 ml Eppendorf tube, tightly closed and keep at -80°C until sectioning on a cryostat.
Cryostat sectioning
- The cryostat chamber should be set to -25°C to -27°C.
- Pre-arrange in a holder and chill on crushed ice super-frost slides.
- Thaw mount 10 µm thick transverse sections, 5–6 per slide.
- Arrange slides in a box and keep at -80°C until fixation for immunohistochemistry.
Fixation
- Place a volume of acetone and a Coplin jar in advance in a -18°C freezer.
- Remove slides from -80°C and place leaning in a flat tray in a fume hood to dry at room temperature for 10 minutes.
- Place slides in the pre-chilled Coplin jar and pour pre-chilled acetone in jar to cover the slides.
- Place Coplin jar in -18°C freezer for 10 min.
- Pour out acetone and allow slides to dry in a fume hood for 10 minutes.
- Fill jar with IHC-phosphate-buffered saline (IHC-PBS) and put in refrigerator (2–8°C) until immunohistochemical processing.
| IHC-PBS (pH 7.4) | |
|---|---|
| Reagent | Concentration (M) |
| Na2HPO4 | 0.016 |
| KH2PO4 | 0.003 |
| NaCl | 0.14 |
Antigen retrieval
- Remove slides from Coplin jar and put on a flat tray and add 200 µl of Antigen Retrieval Solution on top of each slide.
| Antigen Retrieval Solution | |
|---|---|
| Reagent | % of final volume |
| 0.2 M Na2HPO4 | 37.4 |
| 0.2 M NaH2PO4 | 12.5 |
| Methanol | 20 |
| Triton 30%* | 0.2 |
| Hydrogen peroxide 30% | 5 |
| Distilled deionized water | 25 |
- Cover slide with a rectangle of parafilm and leave at room temperature for 25 minutes.
- Rinse off antigen retrieval solution with PBS and arrange slides leaning in a box to dry for 5 minutes.
Staining
- Arrange slides on a flat tray.
- Add your primary antibody in 200 µl Antibody Solution on each slide and cover with parafilm.
| Antibody Solution | |
|---|---|
| Reagent | % of final volume |
| IHC-PBS | 97.65 |
| Triton X-100* | 0.3 |
| Tween-20 | 0.05 |
| Normal serum** | 2 |
*If your primary antibody targets an extracellular protein, reduce the Triton X-100 to 0.05% in both the primary and secondary antibody solutions.
**Use a serum based on the species that your secondary antibody was raised in. For example, if your secondary antibody was raised in donkeys, use normal donkey serum (NDS). Likewise, if your secondary antibody was raised in goats, use normal goat serum (NGS).
- Incubate at room temperature for 1 hour and then refrigerate overnight.
- Wash for 3 x 5 minutes in IHC-PBS.
- Arrange slides on a flat tray and incubate with your secondary antibody (conjugated to a fluorophore or biotin†) in Antibody Solution for 2 hours at room temperature.
†If the secondary antibody is conjugated to biotin, incubate with a solution of streptavidin conjugated to a fluorophore for 1 hour at room temperature. - Wash for 3 x 5 minutes in IHC-PBS.
Detection by fluorescent microscopy
- Stain the sections on the slides with DAPI by placing DAPI solution (5 mg/ml stock solution in deionized water or dimethylformamide (DMF); dilute to 500 nM in IHC-PBS for final use) on each slide. Next, cover each slide with a piece of parafilm to spread the solution evenly on each slide.
- After 2 minutes, remove the parafilm and rinse the slide with 0.5 ml distilled deionized water using a pipette (repeat twice).
- Allow slides to dry in a fume hood for 30 minutes.
- Apply coverslips using the adhesive Immu-MountTM (ShandonTM).
- Dry the slides overnight, protected from light.
- Store at -18°C until they ready to view under the microscope.
Example Data
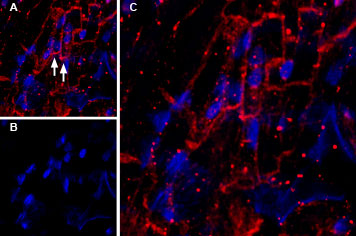
Figure 1: Expression of KCNQ1 in mouse heart. Immunohistochemical staining of mouse heart frozen section using Guinea pig Anti-KCNQ1 Antibody (#AGP-050), (1:200). A. KCNQ1 staining (red) appears in muscle fiber membranes (arrows). B. Nuclei are stained using DAPI counterstain (blue). C. Merged image of panels A and B.
Figure 2: Expression of KCNE2 in mouse heart. Immunohistochemical staining of mouse heart frozen sections using Anti-KCNE2 (MiRP1) Antibody (#APC-054), (red). Immunoreactivity appears as a striated pattern in heart muscle.