When you think about valium, or diazepam, antidepressants and anti-anxiolytics come to mind. If you have a background in pharmacology, you might also think about that old class of drugs, benzodiazepine (BDZs). But what about oxidative stress and the regulation of adrenergic receptors? A new study from Rome’s National Institute of Health provides some fascinating insights into the regulation of β1- and β2-adrenergic receptors (β1- and β2-ARs) during treatment with β-AR agonists like diazepam.
In the course of this study, researchers used selective antibodies directed against an extracellular epitope of β-ARs and flow cytometry to evaluate the cell surface regulation or these receptors in monocytes. They found that diazepam stimulates a modulator of oxidative stress and, in turn, affects cell surface β1-AR density. Moreover, these receptors were found to not be susceptible to agonist-mediated downregulation.
What are β-adrenergic receptors?
β-adrenergic receptors belong to the G-protein-coupled receptor (GPCR) superfamily and respond to catecholamines by activating adenylyl cyclase. GPCRs have complex mechanisms for signal modulation, such as downregulating cell surface receptor density.
The β-ARs subtypes β1-, β2-, and β3-ARs differ in tissue distribution, desensitization mechanisms, and activation of specific signaling pathways, and play diverse roles in the control of cardiac performance and the regulation of immune activity. As such, there is significant interest in understanding the mechanisms regulating β-AR expression to develop more effective therapies for diseases associated with β-AR dysregulation. This makes β-AR expression a prime candidate for study when it comes to developing treatment for diseases arising from β-AR dysregulation.
While much is known about agonist-mediated downregulation of the human β2-adrenergic receptor, less is understood about human β1-AR downregulation. Interestingly, some research suggests that oxidative stress plays a role in regulating the expression of β-AR, including selectively decreasing the expression of β1-adrenergic receptors in mouse cardiomyocytes.
The peripheral benzodiazepine receptor (PBR), AKA mitochondrial translocator protein (TSPO), plays a crucial role in modulating oxidative stress, mitochondrial physiology, and cellular energetics. It also normalizes cellular energetics and the redox balance of pressure-overloaded cardiomyocytes. Consequently, the researchers hypothesized that PBR signaling could be involved in the regulation of cell surface β-AR density.
To test this, they looked at how different ligands of the PBR affect the surface density of β1-and β2-ARs in monocytes.
The right reagents for the job
Antibody specificity is obviously a major concern for all researchers. But when it comes to challenging targets such as GPCRs, having confidence in your antibody is vital. The researchers in this study used antibodies from Alomone Labs to detect the cell surface expression of β1- and β2-adrenergic receptors. In light of concerns regarding the specificity of commercially available anti-βAR antibodies, the researchers conducted preliminary studies to test the suitability of these antibodies to detect the expression of β1- and β2-adrenergic receptors.
In an inspiring feat of scientific ingenuity, the team used antibodies that were directly conjugated to FITC for both flow cytometry and immunofluorescence assays: Anti-β1-Adrenergic Receptor (extracellular)-FITC Antibody (#AAR-023-F) and Anti-β2-Adrenergic Receptor (extracellular)-FITC Antibody (#AAR-016-F). Since the antibodies target extracellular epitopes and have been designed to recognize β1-and β2-adrenergic receptors from mouse, rat, and human samples, they tested them in cells expressing β1-and β2-adrenergic receptor subtypes, such as human THP-1 cells, along with knockout (KO) mouse monocytes that lack β1-AR, β2-AR, or both ARs.
Immunoassays With Anti-β1- and Anti-β2-Adrenergic Receptor Antibodies
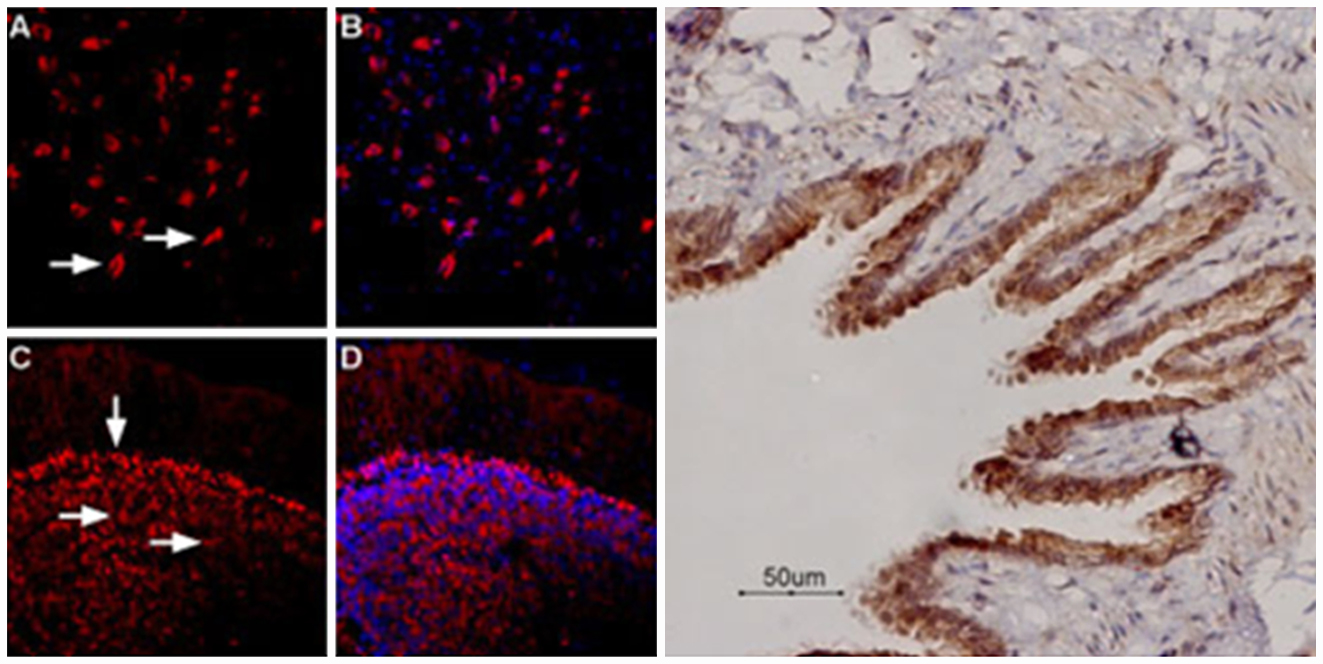
Flow cytometry demonstrated how the antibodies clearly labeled the receptors in the THP-1 cells that endogenously expressed β-ARs on the cell surface. The cell surface β-AR density was consistent with the results reported on the Alomone Labs website, highlighting Alomone’s stellar technical support.
It was now clear that the β-AR antibodies from Alomone Labs bound β-AR, but could they distinguish β1-from β2-ARs? Using peripheral blood monocytes isolated from mice, the team showed that MFI values for β1- and β2-AR expression in wild types were significantly higher than those in KO mouse cells. Alomone’s β-AR antibodies both successfully bound β-AR and differentiated between β1- from β2-AR subtypes. Once antibody validation was completed, it was time to move on to the study’s three main questions: are β1- and β2-ARs differentially regulated by β-AR agonists? Do oxidative stress and PBR/TSPO ligands impact β-AR surface density? What is the mechanism responsible for any changes in β-AR expression?
Are β1- and β2-adrenergic receptors differentially regulated?
Previous research that suggests that β1-ARs are resistant to agonist downregulation compared to β2-Ars. To test this, the researchers stimulated monocytes with epinephrine or isoproterenol, an exogenous catecholamine, for up to 48 hours. Flow cytometry and selective Alomone Labs antibodies against β-ARs revealed that the cell surface density of endogenous β2-adrenergic receptors was reduced, while β1-ARs remained unaffected. This differential regulation of β-ARs could have important implications in the development of therapeutics aimed at targeting β-adrenergic receptors in different tissues.
Does oxidative stress impact β1- and β2-adrenergic receptor density?
Based on previous research showing that oxidative stress, induced via acute hydrogen peroxide or chronic doxorubicin treatment, reduces β1-AR expression in cardiomyocytes, the team wanted to see if oxidative stress also affects β1-adrenergic receptor density.
The researchers treated monocytes with diazepam, a ligand of PBR/TSPO, and found that it led to a decrease in β1-AR density, but no change in the abundance of the β2-ARs. Unlike the β-blocker propranolol, the PBR/TSPO antagonist PK1119 was able to reverse the effect of diazepam, indicating that PBR/TSPO is involved in regulating the cell surface density of β1-ARs.
This elegant finding demonstrates that the effects on β-ARs are not actually mediated by oxidative stress, as no change in the production of oxygen and nitrogen radicals was observed after diazepam treatment.
What is the mechanism behind changes in β1-adrenergic receptor density?
So far, we have seen that β1- and β2-ARs are indeed differentially regulated in monocytes, with β1-AR being more resistant to agonist-mediated downregulation. Conversely, β1-AR density is reduced following exposure to diazepam, an effect we see antagonized by the PBR antagonist PK11195. To deduce a mechanism for these results, the researchers looked at how diazepam affects transcription and receptor synthesis, as well as the monocyte expression of GRK-2, a kinase involved in the desensitization and internalization of β-adrenergic receptors. They also examined the effect of diazepam on the interaction of β1- and β2-adrenergic receptors with Gα-protein subunit or β-arrestin-2.
Interestingly, they found that diazepam had no effect on either mRNA or protein levels of β1-AR or GRK-2, nor did it affect the interaction of β-AR subtypes with Gα-protein or β-arrestin-2. However, the role of PBR/TSPO in regulating β1-AR surface density seems clear. This led the researchers to hypothesize that diazepam may affect surface β1-AR density by altering its recycling to the plasma membrane.
Cardiovascular implications
This study provides important insights into the complex regulatory mechanisms that underlie β-AR behavior and interactions. Considering the importance of β-ARs in cardiovascular treatments (in the form of beta-blockers, for example), these findings suggest that targeting PBR/TSPO may be a promising strategy to modulate β1-AR expression.
This research also underscores the importance of validating your antibodies – even the highly specific ones! Alomone’s antibodies grant you the flexibility to use multiple immunoassays to shed light on the most complex of mechanisms.
Reagents for receptors
Targeting membrane-bound receptors can be challenging. That’s why we’ve been producing and validating a range of antibodies in-house for decades. If you enjoyed the research discussed here, you might be interested in our unique tools:
Antibodies
- Anti-β1-Adrenergic Receptor (extracellular)-FITC Antibody (#AAR-023-F)
- Anti-β2-Adrenergic Receptor (extracellular)-FITC Antibody (#AAR-016-F)
- β-Adrenergic Receptor Antibody Explorer Kit (#AK-500)
Controls
Blockers/Antagonists