Neuropathic pain is chronic and widespread, affecting millions worldwide. Despite its prevalence, the treatments for neuropathic pain can be limited in their efficacy or associated with adverse side effects. But what if there was a way of alleviating neuropathic pain with a natural compound? What if the humble satsuma held the key to pain relief?
You might chuckle at that, but new research has found that narirutin, a flavonoid compound from Citrus unshiu (the satsuma mandarin, or miyagawa mandarin), is very effective at alleviating neuropathic pain in a rat model.
Narirutin: the satsuma’s wonder chemical
Flavonoids have a long history in pain therapy, and turns out, narirutin already has a fascinating track record. Studies have shown how narirutin can be neuroprotective via action on voltage-gated potassium (Kv) channels, stops cancer growth through PI3K/AKT and ERK/MAPK pathways, and its analog, naringenin, is beneficial in acute and chronic rodent pain models.
With this in mind, a team from China interested in neuropathic pain set out to see whether narirutin possesses analgesic properties that could render it a potential therapy for neuropathic pain.
Narirutin reduces the sensation of pain
To assess any general antinociceptive effects of narirutin, the team used the rat spared nerve injury (SNI) model. After SNI surgery and subsequent intrathecal narirutin treatment, they observed a significant reduction in the sensation of pain at an hour post-treatment. Rats also demonstrate reduced sensitivity to cold after narirutin treatment.
With narirutin’s general antinociceptive properties confirmed, it was time to understand the mechanisms behind it.
Narirutin targets Nav1.7 specifically
The team used a veratridine (VTD) assay to first establish that narirutin affects oscillatory (OS) neurons – and changes to OS neurons reflect changes to nociceptors. Previous work had already shown that OS populations are highly correlated with voltage-gated sodium (Nav) 1.7 channels, and so this channel became the team’s main suspected narirutin target.
To unravel this, the researchers used whole cell-patch clamp recordings, along with Alomone’s Tetrodotoxin citrate (#T-550) (TTX) and selective inhibitors of Nav1.8 (PF-05089771 (#P-315) and Nav1.8 A-803467 (#A-105) to isolate Nav currents. These data showed how narirutin primarily affects TTX-sensitive currents, blocking Nav 1.7 in both small-diameter dorsal root ganglion (DRG) nociceptive neurons and HEK 293 cells. Interestingly, narirutin had no effect on TTX-resistant channels like Nav 1.8 and Nav 1.9 (we recently highlighted how spider venom reveals crosstalk between Nav channels in relation to pain).
Narirutin also downregulates Nav1.7 expression
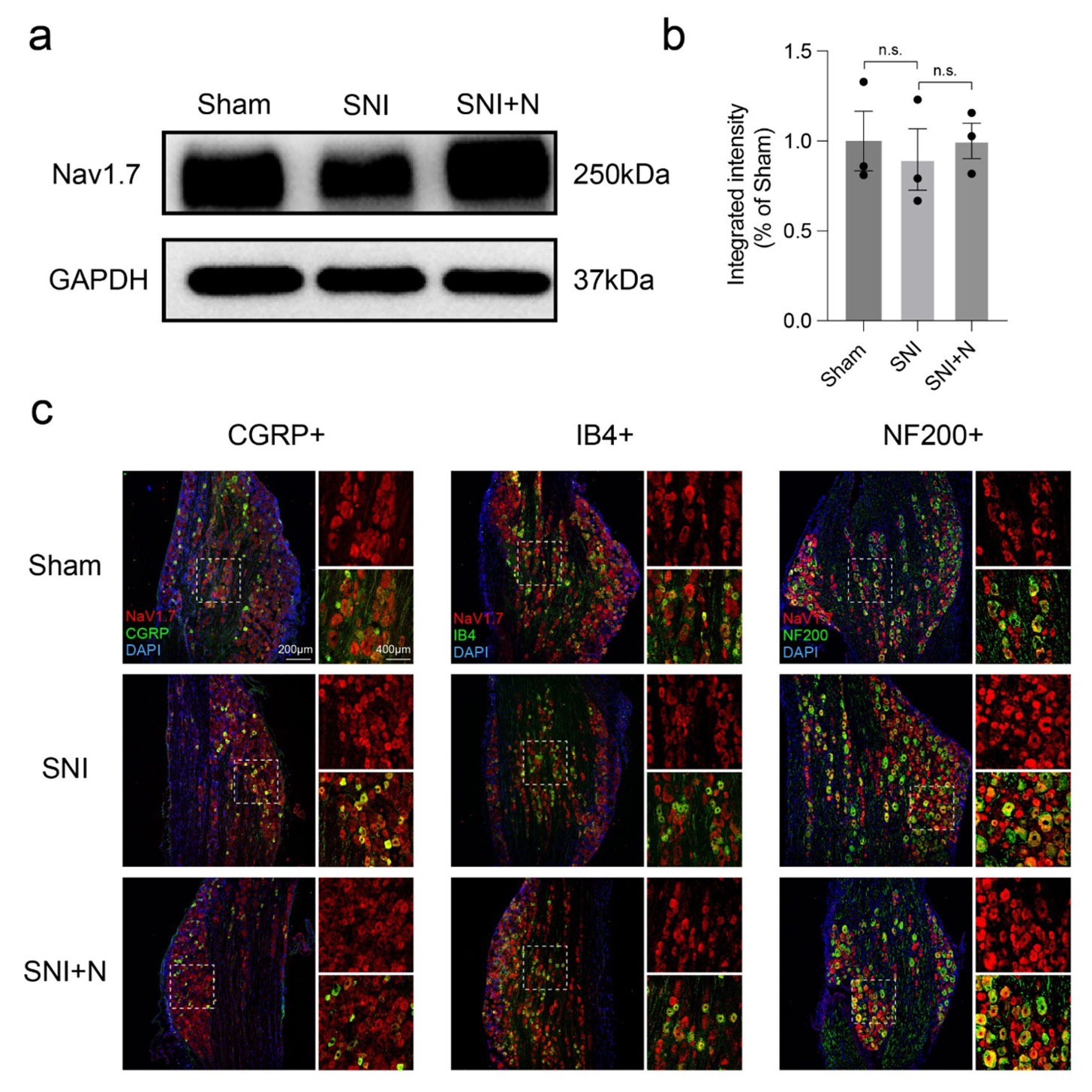
In addition to inhibiting Nav1.7, the team wanted to see whether narirutin affects Nav1.7 expression. To test this, they looked at DRGs in SNI rats. While a Western blot using Alomone’s Anti-NaV1.7 (SCN9A) Antibody (#ASC-008) revealed no significant difference in Nav1.7 expression between sham (control) and treated rats, immunohistochemistry indicated that Nav1.7 expression varies between different types of neurons after SNI.
When separately co-immunostaining Nav1.7 with CGRP, IB4 (for unmyelinated peptidergic or non-peptidergic sensory neurons), and NF200 (for myelinated neurons), they observed an increase in Nav1.7-positive cells in CGRP-labeled neurons (Figure 1). When treating with narirutin, this increase in Nav1.7-positive cells is limited – something not seen in the IB4- or NF200-labeled neurons. This suggests narirutin can downregulate Nav1.7 expression in CGRP+ DRG nociceptive neurons.
Expression of Nav1.7 in different types of DRG sensory neurons
Pain relief in a satsuma
This interesting work elegantly shows how a flavonoid from a satsuma, narirutin, selectively inhibits Nav1.7 and downregulates its expression in certain sensory neurons. There’s still more work needed to fully explain the analgesic properties of this natural compound, but at the very least this research has identified narirutin as a potential therapeutic treatment for neuropathic pain.
- Tetrodotoxin citrate (#T-550)
- PF-05089771 (#P-315)
- A-803467 (#A-105)
- Anti-NaV1.7 (SCN9A) Antibody (#ASC-008)
- Guinea pig Anti-NaV1.7 (SCN9A) Antibody (#ASC-008-GP)
- Anti-NaV1.7 (SCN9A)-ATTO Fluor-633 Antibody (#ASC-008-FR)

If you need something more bespoke, like a custom conjugation, novel toxin, or just a bulk order, you can always use our Custom Services.
