The muscarinic receptors belong to the G-protein coupled receptors (GPCR) family. In mammals, at least five different muscarinic acetylcholine receptor subtypes (mAChRs; m1 through m5) are known to be widely expressed and distributed in different tissues from different species. They mediate distinct physiological functions according to their location and receptor subtype. Here, we summarize selected published uses of Alomone Labs’ anti-muscarinic antibodies, which contribute to the understanding of the physiological aspects of mAChR and their mechanisms of action.
Introduction
Acetylcholine (Ach), the major neurotransmitter in the central and peripheral nervous systems acts through two kinds of receptors: ionotropic receptors, which consist of rapidly activated ion channels and metabotropic receptors. The metabotropic receptors regulate ion channels and many physiological processes through their binding and activation of G proteins. The ACh metabotropic receptors are also called muscarinic receptors (mAChRs), and are widely distributed in the plasma membranes of certain neurons and other mammalian cells.
Five subtypes of muscarinic receptors have been determined, m1 through m51. They vary in their distribution and their affinity to various G proteins, with some correlation according to the receptor subtype. As a member of the G-protein coupled receptors (GPCR) family, they mediate most of the physiological responses to hormones, neurotransmitters and environmental stimulants. Like other G-protein coupled receptors, they have a seven transmembrane domain with an extracellular N-terminus.
The use of specific antibodies directed specifically to each one of the receptors can assist in elucidating the physiological importance of these receptors. Three antibodies are offered by Alomone Labs: Anti-m1 (#AMR-001), Anti-m2 (#AMR-002) and Anti-m3 antibodies (#AMR-006).
M1 Muscarinic Receptors
M1 muscarinic receptors (m1) mediate slow EPSP (excitatory postsynaptic potential) at the ganglion in the postganglionic nerve, and are common in exocrine glands and in the CNS. They are predominantly found acting via G proteins of class Gq. The distribution of m1 in RGS9-2 transfected CHO cells was monitored using Anti-m1 antibody (#AMR-001) in immunohistochemical analysis. The research showed colocalization of both m1 and RGS9-2 in these cells2.
Immunohistochemical studies showed that in the rat cortex and hippocampus the Slack (Slo2.2) and Slick (Slo2.1) ion channels, members of the mammalian Slo K+ channel gene family, colocalize with m13 (Figure 1).
Immunocytochemical experiments performed in additional work, indicated that the m1 muscarinic receptor subtype is present in both rat and mudpuppy taste cells4.
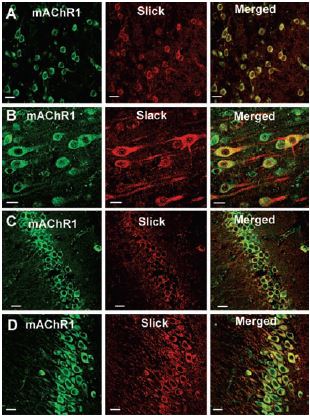
Top 12 panels show colocalization of Slick or Slack with mAChR1, using Anti-m1 antibody (#AMR-001) in sections of rat cortex (A, frontal cortex; B, layer V of frontal cortex) and in hippocampus (C, CA1 region; D, CA3 region). Slick and Slack immunoreactivity was localized with Cy3 (red), and muscarinic receptor immunoreactivity was visualized using Alexa Fluor 488 secondary antibody (green). On the overlaid images, regions of colocalization appear orange to yellow. Scale bar, 20 µm.
Adapted from reference 3 with permission of The Society for Neuroscience.
M2 Muscarinic Receptors
M2 muscarinic receptors (m2) are expressed in the heart, where they act to slow the heart rate down to normal sinus rhythm by slowing the speed of depolarization after stimulatory actions of the parasympathetic nervous system. They also reduce contractile forces of the atrial cardiac muscle, and reduce conduction velocity of the atrioventricular node (AV node). M2 muscarinic receptors act via G proteins of class Gi. Immunoprecipitation studies using Anti-m2 antibody (#AMR-002) showed that m2 is not associated with the native rat atrial KACh channels complex, composed of GIRK1-Gβ complex, GIRK2/3, PKAc, PP1, PP2A, and RACK1 proteins5.
Additional work using confocal microscopy, showed that Kv2.1 K+ channel expression is enriched at synapses formed by cholinergic C-terminals, and that the Kv2.1 subunit-containing channels are colocalized with postsynaptic m26 (Figure 2). Anti-m2 antibody was also used in immunoprecipitation studies to monitor the effect of receptor stimulation on m2 protein levels in atrial membrane. It was demonstrated that G protein-regulated inwardly rectifying K+ (GIRK) channels can operate as dynamic integrators of α-Adrenergic and cholinergic signals in rat atrial myocytes7. It was also demonstrated in HEK293 cells, stably expressing m2 receptors (using immunocytochemical studies) that the internalization of m2 is modulated by the activation of heterotrimeric G proteins and is directed mainly to the Golgi8 (Figure 3). Immunofluoresence showed that expression of m2 is widespread in sections of E17 cerebral rat cortex embryos. M2 receptors were found to be distributed in the ventricular, subventricular and cortical plate zones, indicating that these cells are proliferating neuroepithelial cells9 (Figure 4).
In resting cardiomyocytes, m2 and β1-AR Adrenergic Receptors have a similar membrane distribution, which was demonstrated by western blot analysis. In contrast, β2-ARs and m2 largely segregate to separate membrane subdomains10.
Using Anti-m2 antibody in immunoprecipitation studies, the collision-coupling mechanism between m2 and G proteins was assessed. Results of this study suggest a stoichiometric coupling between the G protein and GIRK as the effectors in Xenopus oocytes11.
Additional work proposed, by western blot analysis, that the acidic amino acids at sites A1 and A2 in m2 play important roles in agonistdependent phosphorylation at sites P2 and P1, respectively, and also play an important role in interactions of arrestin with m212.
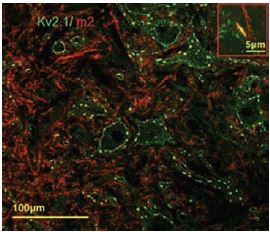
Postsynaptic m2 muscarinic receptor (red), identified by using the Anti-m2 antibody (#AMR-002) is colocalized with surface membrane Kv2.1-immunoreactivity (KV2.1-IR) (green). At low and high power (inset), the two channels of fluorescence signal overlap almost completely at the locations of large clusters of Kv2.1 (yellow, inset).
Adapted from reference 6 with permission of Blackwell Publishing Ltd.
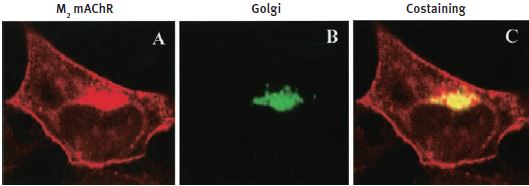
HEK293 cells stably expressing m2 mAChRs were fixed and stained with Anti-m2 antibody (#AMR-002) (A) and anti-Golgi zone antibodies (B) and visualized with TRITC-conjugated anti-rat antibodies and FITC-conjugated anti-mouse antibodies. C) An overlay of the corresponding m2 mAChR and Golgi zone images (yellow color indicates colocalization).
Adapted from reference 8 with permission of the American Society for Pharmacology & Experimental Therapeutics.

Nissl staining (A) and immunofluorescence using Anti-m2 antibody (#AMR-002) (B) show a widespread distribution of m2 AChR-immunoreactive cells in the vz and sv zones and cortical plate (CP) of coronal sections of E17 rat cortex. Scale bar, 150 µm.
Adapted from reference 9 with permission of The Society for Neuroscience.
M3 Muscarinic Receptors
M3 muscarinic receptors (m3) are located in the smooth muscles of the blood vessels, as well as in the lungs. M3 receptors act via G proteins of class Gq. Anti-m3 antibody (#AMR-006) was used, in immunocytochemistry studies, to confirm that mesencephalic GABAergic neurons can be regulated directly through muscarinic receptors13 (Figure 5).
Additionally, m3 is detected using immunocytochemistry analysis at the plasma membrane of human ASM (airway smooth muscle) cells containing caveolin-1. Caveolin-1 contributes to regulation of [Ca2+]i responses to agonists in these cells14 (Figure 6).
M4 Muscarinic Receptors
M4 muscarinic receptors (m4) are found mainly in the CNS. These receptors act via G proteins of class Gi.
The M5 Muscarinic Receptors
The M5 muscarinic receptors (m5) distribution s not well known. Like the m1 and m3 receptors, m5 receptors are coupled with G proteins of class Gq.
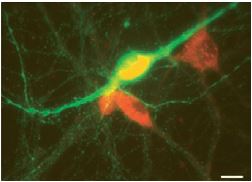
Image showing the result of a double-labelling immunofluorescence experiment performed using Anti-m3 antibody (#AMR-006) (red) and a tyrosine hydroxylase (TH) antibody (green). Scale bar, 15 µm. M3-like immunoreactivity is observed in non-TH (presumed GABAergic) neurons.
Adapted from reference 13 with permission of Blackwell Publishing Ltd.
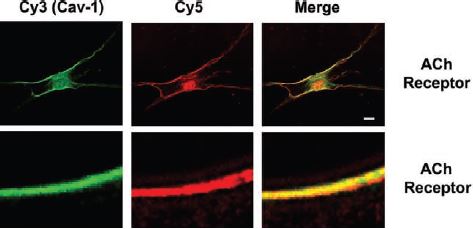
Two-color immunocytochemistry and laser scanning confocal microscopy were used to localize caveolin-1 (green, Cy3) predominantly to the plasma membrane of ASM cells. Caveolin-1 colocalized with several intracellular Ca2+ concentration ([Ca2+]i)-regulatory proteins (red, Cy5), including receptors for Ach (m3 receptor) using Anti-m3 antibody (#AMR-006) (shown at X1,000 magnification). Scale bar, 1 µm, except in top row (10 µm).
Adapted from reference 14 with permission of the American Physiological Society.
References
- Wess, J. (1993) Life Sci. 53, 1447.
- Kovoor, A. et al. (2005) J. Neurosci. 25, 2157.
- Santi, C.M. et al. (2006) J. Neurosci. 26, 5059.
- Ogura, T. (2002) J. Neurophysiol. 87, 2643.
- Nikolov, E.N. and Ivanova-Nikolova, T.T. (2004) J. Biol. Chem. 279, 23630.
- Muennich, E.A.L. and Fyffe, R.E.W. (2003) J. Physiol. 554, 673.
- Nikolov, E.N. and Ivanova-Nikolova, T.T. (2007) J. Biol. Chem. 282, 28669.
- Roseberry, A.G. et al. (2001) Mol. Pharmacol. 59, 1256.
- Li, B.S. et al. (2001) J. Neurosci. 21, 1569.
- Rybin, V.O. et al. (2000) J. Biol. Chem. 275, 41447.
- Vorobiov, D. et al. (2000) J. Biol. Chem. 275, 4166.
- Lee, K.B. et al. (2000) J. Biol. Chem. 275, 35767.
- Michel, F.J. et al. (2004) J. Physiol. 556, 429.
- Prakash, Y.S. et al. (2007) Am. J. Physiol. 293, L1118.