You want to know that your primary antibody binds the target protein it’s supposed to. Blocking peptides are one of several controls you should use to gain more insight into antibody specificity.
A blocking peptide is the original antigen we use for immunization during polyclonal antibody generation. You can easily use a blocking peptide as a negative control alongside your immunoassay to help determine whether your antibody binds the protein it was developed to target. Ultimately, getting these controls right in the first place means less time troubleshooting your assay and more time looking at your results.
You can find blocking peptides for every polyclonal antibody we offer. Since you might not always have access to a knockout cell line to validate your antibodies, our blocking peptides are a great starting place and are readily available.
You’ll receive your blocking peptides as a convenient and stable lyophilized powder after we subject them to:
- Peptide confirmation by amino acid analysis and mass spectrometry
- Lot-to-lot quality control by western blot
How and why to use blocking peptides
As the original antigen used during immunization, blocking peptides are great to include in your controls. Sometimes called “immunizing peptides” or “negative control antigens,” a blocking peptide works by competing with, or blocking, the primary antibody.
Blocking peptides are a great tool as part of several methods. Controls like this not only help you to understand whether an antibody binds its intended target, but whether your assay is working as expected. These are essential points for when you need to corroborate results between experiments.
It’s important to remember that while blocking peptides can give you insight into specificity, they do not provide information about selectivity. A blocking peptide is a reagent control (like a no-primary or isotype control), but you should also use antigen controls as well, like a cell or tissue type you know definitely does (positive control) or does not (negative control) express the protein of interest.
An antibody blocked with a blocking peptide should ideally produce no signal when added to your tissue or cell sample. It’s this absence of staining when the blocked antibody is used that is likely to indicate specific binding. Positive results in the blocking peptide control likely mean the antibody is binding to a protein besides the target.
No Blocking Peptide
Incubate with blocking peptide
Primary antibody added to samples


Washed and secondary antibody added


Washed and detected


Blocking peptide has bound, or “blocked”, the primary antibody. No signal is seen in the negative control. This can help to show antibody specificity in conjunction with additional controls.
If you’d like specific experimental details, you can find our blocking peptide protocols for western blot and ICC/IHC below.
Blockin Peptides in action
In Figure 1, you can see immunocytochemistry (ICC) data from mouse mCCDcl1 kidney cells using Anti-TRPV4 Antibody (ACC-034), (green). There is no TRPV4 staining at all when the antibody is preincubated with the TRPV4 Blocking Peptide (BLP-CC034) (top right panel, “TRPV4+ BP”).
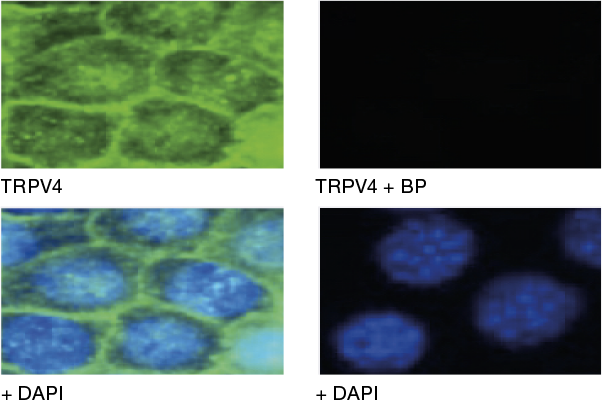
ICC using Anti-TRPV4 Antibody (ACC-034) with blocking peptide. Adapted from Li, Y. et al. (2016) PLoS ONE 11, e0155006 with permission of PLoS.
Blocking peptides also work with western blot (WB). In Figure 2, human plasmacytoma (INA-6) cells, human mesenchymal stem cell (MSC), and human breast cancer cell lysates are stained with Anti-KISS1 Receptor (extracellular) Antibody (AKR-001) (upper panels). The band observed disappears when the antibody is preincubated with the KISS1 Receptor (extracellular) Blocking Peptide (BLP-KR001) (bottom panel, “Blocking +”).
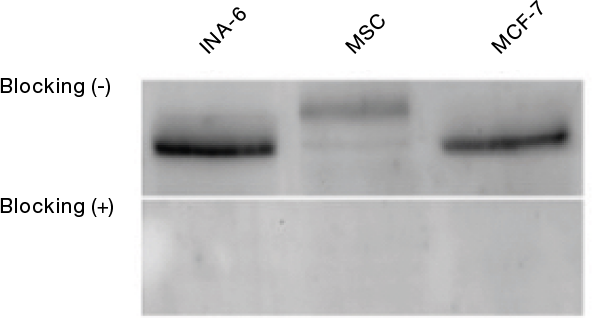
WB using Anti-KISS1 Receptor (extracellular) Antibody (AKR-001) with the antigen blocking peptide for KISS1R. Adapted from Dotterweich, J. et al. (2016) PLoS ONE 11, e0155087 with permission of PLoS.
