Long understood to play a protective role in adult hearts, particularly in cases of ischemia and reperfusion (IR) injury, the function large-conductance calcium and voltage-activated potassium channels (BKCa channels) in neonatal cardiac tissues has remained largely elusive. Until recently, most studies focused on the role of BKCa channels in adult cardiomyocytes, often overlooking their presence or absence in neonatal hearts. However, a new study in Nature’s Cell Death Discovery changes that by confirming – for the first time – that BKCa channels exist in the plasma membrane of neonatal cardiomyocytes (NCMs). The researchers went beyond mere observation to reveal the impact of these channels on neonatal heart function with implication for dealing with cardiac injury.
BKCa Channels in Neonatal Cardiomyocytes: A Shift in Localization
While BKCa channels are predominantly localized in the mitochondria of adult mouse and rat ventricular cardiomyocytes, the story is a somewhat different when it comes to NCMs. To demonstrate this, the scientists in this new paper used a specific anti-BKCa antibody from Alomone Labs (Anti-KCNMA1 (KCa1.1) (1097-1196) Antibody (#APC-021)) to observe the localization of these channels in NCMs from both rats and mice. They found that, much like in adults, these channels were largely localized to the mitochondria (Figure 1), which are the energy-producing centers of the cells.
What sets NCMs apart, however, is the significant presence of BKCa channels in the plasma membrane. In adult ventricular cardiomyocytes from rats and mice, just 12% of BKCa channels localized to the plasma membrane. In stark contrast, NCMs showed a robust 26–32% localization to the plasma membrane. This shift in localization between NCMs and adult cardiomyocytes could point to evolving functional roles of BKCa channels as the heart cells mature.
ICC Reveals BKCa Channels in the Plasma Membrane of Neonatal Cardiomyocytes

Age-Dependent Shifts in BKCa Channel Localization
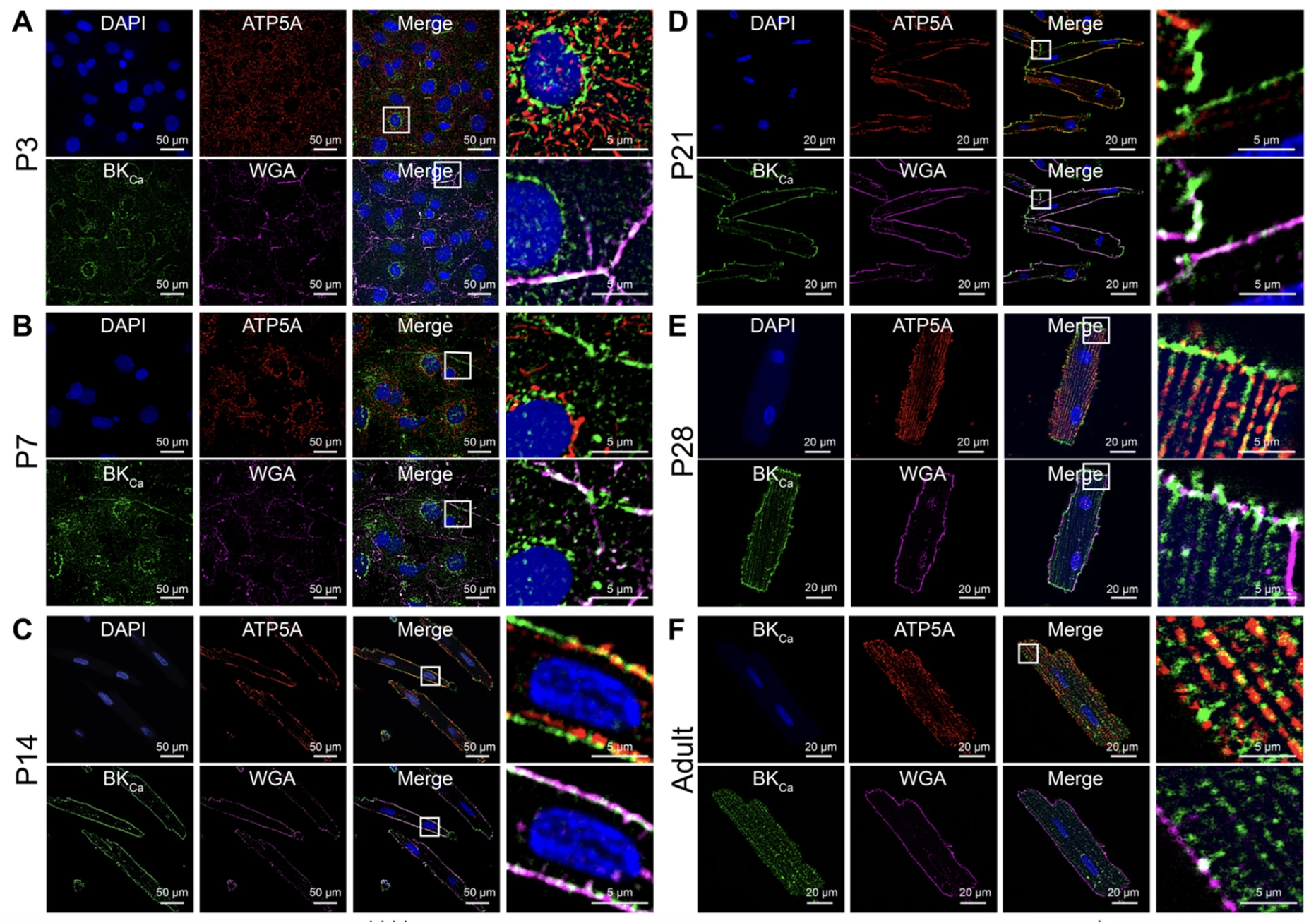
Delving deeper into BKCa channel localization, they set out to chart its progression across various developmental stages of cardiomyocytes. The team studied cells at several stages – P3, P7, P14, P21, P28 days, and adulthood – in rats. Consistent with earlier findings, BKCa channels in NCMs appeared in both the plasma membrane and the mitochondria (Figure 2). Intriguingly, protein proximity index analysis revealed that as the cardiomyocytes age, BKCa‘s affinity for mitochondrial localization gradually ramps up, while its presence in the plasma membrane diminishes.
Here’s where a specific molecular variant, known as the C-terminus DEC splice variant (BKCa DEC), comes in. The scientists observed a surge in the expression of this variant from the P3 stage to adulthood. This uptick coincides with the increasingly mitochondrial-centric location of BKCa channels in maturing cardiomyocytes.
During cardiac development, electrical conduction and energy metabolism are the two significant changes that occur. The presence of BKCa channels in the plasma membrane of neonatal cells could be a transitional phase. As cardiac cells mature, these channels may migrate to their destined organelles like mitochondria, reflecting changes in the metabolic needs of the heart muscle.
The Changing BKCa Channel Localization During Cardiomyocyte Development
Neonatal Hearts Respond Differently to BKCa Channel Activation
Turning to the practical implications, researchers sought to understand how BKCa channel activation impacts the risk of myocardial infarction, particularly in neonatal hearts. Previous studies have demonstrated that activating these channels can shield adult hearts from IR injury. Astonishingly, the team found that the script flips for neonatal hearts. When 6-day-old rat hearts were exposed to a BKCa channel activator, NS1619, the resulting infarct size ballooned by 10% compared to the control. Conversely, inhibiting the BKCa channels with a cell-impermeable BKCa inhibitor, iberiotoxin (IbTx), reduced the infarct size by 16%.
Furthermore, the team recorded potassium currents in nMCMs to confirm the functional activity of these BKCa channels on the plasma membrane. Results revealed that potassium currents were significantly reduced by 33% when adding IbTx. Clearly, for neonates, the activation of BKCa channels amplifies cardiac damage post-IR injury, whereas inhibition offers a protective effect.
Activating BKCa channels in NCMs neither affected membrane depolarization nor shortened the duration of action potentials. Instead, it led to delayed repolarization and significantly prolonged the action potential. Inhibiting these channels, however, showed no such effects, which suggests that in normal physiological conditions, these channels remain inactive in NCMs.
Role in Apoptosis: A Twist in the Tale
Building upon this, researchers explored the role of BKCa channels in apoptosis following hypoxia-reoxygenation (HR) injuries. The researchers used IbTx here again and saw the number of apoptotic cells was nearly identical between the control and the BKCa inhibitor group after HR injury. However, treatment with the BKCa activator, NS-1619, led to a dramatic increase in apoptotic cells – more than double the control rate.
This counterintuitive outcome upends previous assumptions, revealing that BKCa activation, rather than providing protection, actually induces greater cardiac vulnerability and apoptosis in neonatal cells. It’s a tale of two life stages, where the same molecule can play the hero or the villain, depending on the age of the heart it interacts with.
Implications for Cardiac Development and Protection
The study hints at a complex physiological role for BKCa channels in young hearts, potentially offering new strategies for cardioprotection in neonates. The study’s findings also have implications for pharmacological treatment in pediatric cardiology, especially in conditions like dilated cardiomyopathy. In adults, activating BKCa channels tends to be protective against ischemic injuries. However, the data here suggest that activating these channels in neonates could be detrimental, likely due to their localization in the plasma membrane. This might explain why pediatric and adult responses to pharmacological interventions diverge, urging reconsideration in treatment plans for younger populations.
In summary, inhibiting BKCa channels in the plasma membrane of neonatal cardiomyocytes shields these young hearts from IR damage. Moreover, the differing roles of BKCa channels in protecting adult versus neonatal hearts likely stem from their distinct locations within the cell. It also raises vital questions about age-specific treatment protocols, which could lead to more nuanced, effective therapies.
Antibodies
- Anti-KCNMA1 (KCa1.1) (1097-1196) Antibody (#APC-021)
- Anti-KCNMA1 (KCa1.1) (extracellular) Antibody (#APC-151)
- Guinea pig Anti-KCNMA1 (KCa1.1) (1097-1196) Antibody (#APC-021-GP)
Pharmacological Tools
- Iberiotoxin (#STI-400) – A Potent and Specific Blocker of KCa1.1 K+ Channels
- NS-1619 (#N-105) – Selective and Potent Enhancer of KCa1.1 Channels and an Inhibitor of SERCA Pumps
Explorer Kits
- BKCa Channel Antibody Explorer Kit (#AK-217)
- Mitochondrial Ion Channel Antibody Explorer Kit (#AK-535)
- KCa1.1 Channel Modulator Explorer Kit (#EK-132)
- KCa Channel Blocker Explorer Kit (#EK-101)
- KCa Channel Opener Explorer Kit (#EK-201)

