Overview
- Peptide DPKSAAQNSKPRLSFSTK(C), corresponding to residues 8-25 of human KCNK2 (Accession O95069). Intracellular, N-terminus.
 Western blot analysis of rat brain membranes:1. Anti-KCNK2 (TREK-1) Antibody (#APC-047), (1:200).
Western blot analysis of rat brain membranes:1. Anti-KCNK2 (TREK-1) Antibody (#APC-047), (1:200).
2. Anti-KCNK2 (TREK-1) Antibody, preincubated with KCNK2/TREK-1 Blocking Peptide (#BLP-PC047).
- Rat hippocampus sections (Banerjee, A. et al. (2016) J. Neurochem. 138, 265.).
- Mouse primary cortical astrocytes (1:100) (Hwang, E.M. et al. (2014) Nat. Commun. 5, 3227.).
- Maingret, F. et al. (1999) J. Biol. Chem. 274, 26691.
- Lesage, F. and Lazdunski, M. (2000) Am. J. Physiol. Renal Physiol. 279, F793.
- Kim, D. (2003) Trends Pharmacol. Sci. 24, 648.
- Heurteaux, C. et al. (2006) Nat. Neurosci. 9, 1134.
- Woo, D.H. et al. (2012) Cell 151, 25.
KCNK2 (also named TWIK-related K+ channel, TREK-1 or K2P2.1) is a member of the 2-pore (2P) domain K+ channels family that includes at least 16 members. These channels show little time- or voltage-dependence and are considered to be “leak” or “background” K+ channels, thereby generating background currents which help set the membrane resting potential and cell excitation.
The K2P channels have a signature topology that includes four transmembrane domains and two pore domains with intracellular N- and C- termini.
K2P channels are regulated by diverse physical and chemical stimuli including temperature, changes in intracellular pH, mechanical stretch, inhalation anesthetics, etc. The channels can then be subclassified based in their specific activators. KCNK2 can be integrated to a K2P subfamily that includes K2P4.1 (TRAAK) and K2P10.1 (TREK2) that are activated by intracellular unsaturated fatty acids such as arachidonic acid, lysophosphatidic acid and mechanical stretch. In addition, KCNK2 can also be activated by general anesthetics such as halothane and chloroform and intracellular acidification.
KCNK2 expression in humans is largely restricted to the brain with some expression in ovary and small intestine while KCNK2 expression in rodents is more widespread.
KCNK2 has an important role in mood regulation, as knockout mice show resistance to depression, suggesting that KCNK2 may be a potential target for anti-depressants.
KCNK2 is involved in the fast release of glutamate from astrocytes. It requires the activation of Gαi, dissociation of Gβγ, followed by the opening of the glutamate permeable KCNK2 (TREK-1) K+ channel through its interaction with Gβγ. For this purpose, KCNK2 is localized at the cell surface of the cell body and processes of astrocytes.
Application key:
Species reactivity key:
Anti-KCNK2 (TREK-1) Antibody (#APC-047) is a highly specific antibody directed against an epitope of the human protein. The antibody can be used in western blot, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize TREK-1 from mouse, rat, and human samples.
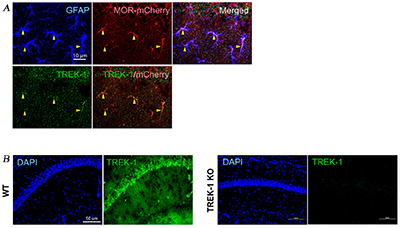
Knockout validation of Anti-KCNK2 (TREK-1) Antibody in mouse hippocampus and expression of TREK-1 in mouse astrocytes. Immunohistochemical staining of mouse brain sections using Anti-KCNK2 (TREK-1) Antibody (#APC-047). A. TREK-1 staining (green) co-localizes with µ-opioid receptor (red) in astrocytes in CA1 region. B. TREK-1 expression (green) in mouse hippocampus (left panel). Lack of TREK-1 staining in TREK-1-/- mice confirms antibody specificity (right panel).Adapted from Woo, D.H. et al. (2018) Front. Cell. Neurosci. 12, 319. with permission of Frontiers.
Applications
Citations
 Expression of TREK-1 in rat hippocampus.Immunohistochemical staining of rat brain sections using Anti-KCNK2 (TREK-1) Antibody (#APC-047). TREK-1 staining (red) in the hippocampus is detected in the stratum radiatum, in the neuropil. Similar diffused staining is observed for GLAST. GFAP staining, the astrocyte marker is also shown.
Expression of TREK-1 in rat hippocampus.Immunohistochemical staining of rat brain sections using Anti-KCNK2 (TREK-1) Antibody (#APC-047). TREK-1 staining (red) in the hippocampus is detected in the stratum radiatum, in the neuropil. Similar diffused staining is observed for GLAST. GFAP staining, the astrocyte marker is also shown.
Adapted from Zhou, M. et al. (2009) J. Neurosci. 29, 8551. with permission of the Society for Neuroscience.
- Mouse spinal cord lysate. Also tested in TREK-1-/- mice.
Fang, Y. et al. (2017) J. Neurochem. 141, 236. - Rat astrocyte lysate (1:100).
Banerjee, A. et al. (2016) J. Neurochem. 138, 265. - Rat astrocyte lysate.
Wu, X. et al. (2013) J. Mol. Neurosci. 49, 499. - Mouse myometrium lysate.
Monaghan, K. et al. (2011) J. Physiol. 589, 1221. - Human adrenocortical carcinoma H295R cells.
Brenner, T. and O'Shaughnessy, K.M. (2008) Am. J. Physiol. 295, E1480.
- Mouse brain sections. Also tested in TREK-1-/- mice.
Woo, D.H. et al. (2018) Front. Cell. Neurosci. 12, 319. - Mouse brain sections.
Cai, Y. et al. (2017) Neurobiol. Learn. Mem. 145, 199. - Mouse spinal cord sections.
Fang, Y. et al. (2017) J. Neurochem. 141, 236. - Rat Hippocampus sections.
Banerjee, A. et al. (2016) J. Neurochem. 138, 265. - Mouse myometrium sections.
Monaghan, K. et al. (2011) J. Physiol. 589, 1221. - Rat brain sections.
Zhou, M. et al. (2009) J. Neurosci. 29, 8551.
- Rat astrocytes.
Banerjee, A. et al. (2016) J. Neurochem. 138, 265. - Mouse primary cortical astrocytes (1:100).
Hwang, E.M. et al. (2014) Nat. Commun. 5, 3227. - Rat astrocyte culture.
Wu, X. et al. (2013) J. Mol. Neurosci. 49, 499.
- Du, Y. et al. (2016) Front. Cell. Neurosci. 10, 13.
- de la Pena, E. et al. (2012) PLoS ONE 7, e52475.
- Woo, D.H. et al. (2012) Cell 151, 25.
- Lembrechts, R. et al. (2011) Histochem. Cell. Biol. 136, 371.
- Baker, S.A. et al. (2010) J. Urol. 183, 793.
- Mazella, J. et al. (2010) PLoS Biol. 8, e1000355.
- Zhou, M. et al. (2009) J. Neurosci. 29, 8551.
- Brenner, A. et al. (2008) Am. J. Physiol. Endocrinol. Metab. 295, E1480.
- Voloshyna, I. et al. (2008) Cancer Res. 68, 1197.
- Magra, M. et al. (2007) Am. J. Physiol. Cell Physiol. 292, C1053.
- Zhao, M. et al. (2007) J. Neurosci. 27, 12025.
- Gardener, M.J. et al. (2004) Br. J. Pharmacol. 142, 192.
- Bai, X. et al. (2005) Reproduction 129, 525.
- Murbartian, J. et al. (2005) J. Biol. Chem. 280, 30175.
- Eaton, M.J. et al. (2004) NeuroReport 15, 321.
