Strokes are a leading cause of death and disability worldwide, with ischemic strokes accounting for about 85% of all stroke cases. The search for therapeutic strategies to aid recovery continues to be a pressing challenge. A new study focusing on targeting acid-sensing ion channel 1a (ASIC1a) has unveiled promising results in promoting neural progenitor cell (NPC) migration and neurogenesis.
The Role of ASIC1a in Ischemic Strokes
NPCs, which can migrate from the subventricular zone (SVZ) to the infarct area, play a vital role in promoting neurogenesis. However, in some instances, these cells differentiate into astrocytes and form glial scars, rather than aiding recovery. Targeting factors that inhibit NPC migration could serve as a promising therapeutic strategy.
Ischemic stroke causes microenvironmental acidosis, leading to an accumulation of extracellular protons. This acidosis activates ASICs, specifically ASIC1a, which can result in further damage to the brain tissue around the infarct area as ASIC1a is predominantly expressed in CNS neurons. Previous research has shown that activating ASIC1a contributes to ischemic injury, whereas blocking ASIC1a can reduce damage after a stroke.
ASIC1a is Expressed in NPCs
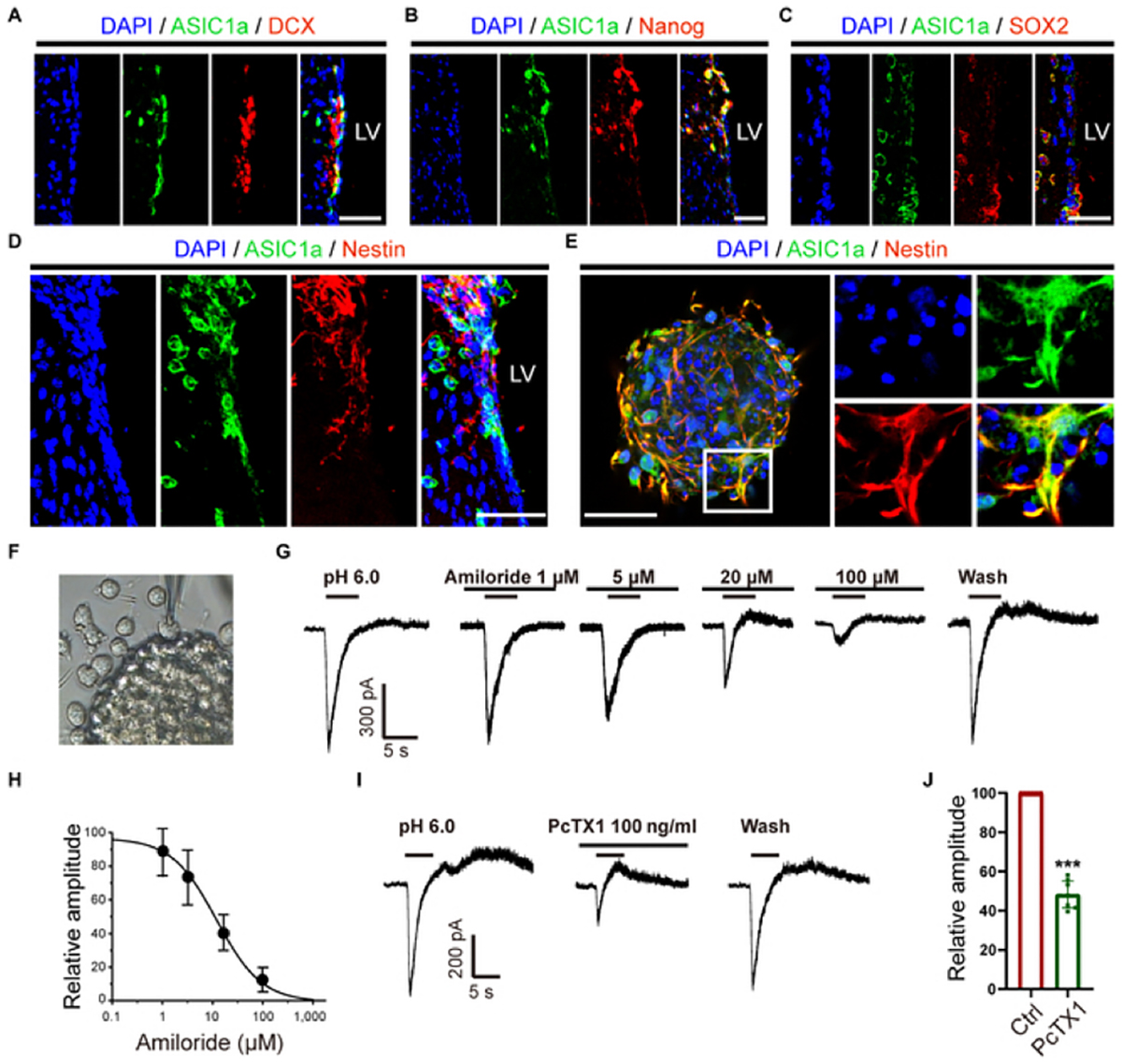
In this new study, the researchers set out to examine ASIC1a expression. They confirmed that ASIC1a (immunolabeled with an Anti-ASIC1 Antibody (#ASC-014) from Alomone Labs) was expressed in NPCs (Figure 1) and also colocalized with doublecortin (DCX), Nanog, SOX2, and Nestin.
In the cultured adult NPCs, the cells showed multipotency by differentiating into various cells – ASIC1a was co-labeled with Nestin+ cells. Whole-cell patch clamp techniques demonstrated that large transient inward currents were activated by extracellular acidosis at pH 6.0, and that these could be blocked by amiloride and psalmotoxin 1 (PcTX1) in a dose-dependent manner, confirming sensitivity to ASIC inhibitors. The inhibitory effects of the inhibitors were almost completely reversed after washout, providing further evidence of ASIC currents. Collectively, these findings affirm that ASIC1a is expressed in NPCs around the lateral ventricle (LV) in adult mouse brains, and forms functional channels that are sensitive to acidic stimulation.
ASIC1a Expression in Primary NPCs From C57BL/6 Mice
Pharmacological Inhibition and Deletion of ASIC1a
The researchers found that ASIC1a knockdown or the application of ASIC1a antagonists facilitated NPC migration and neurogenesis, promoting functional recovery. In particular, the study demonstrated that ASIC1a deletion promotes functional recovery by facilitating NPC migration in mice. Moreover, ASIC1a activation inhibits NPC differentiation into neurons, and pharmacological inhibition of ASIC1a improves NPC migration and behavioral recovery. These findings underscore the significant role of ASIC1a in regulating NPCs.
ASIC1a and RhoA: A Crucial Interaction
The researchers identified RhoA as a pivotal downstream effector when activating ASIC1a under acidic conditions. The researchers also discovered that, under acidic conditions (pH 6.5), filopodia formation decreased, hampering NPC migration and neurogenesis. Conversely, in ASIC1a−/− NPCs, the percentage of filopodia formation and leading processes increased.
The molecular mechanisms were further investigated using RNA-seq, identifying 162 differentially expressed genes and highlighting ASIC1, RhoA, and Rac1 as hub genes affecting NPC migration and differentiation. The expression of active RhoA was notably increased under acidic conditions, while ASIC1a knockout reversed this effect.
Subsequent experiments using a specific RhoA inhibitor (Y27632) and small interfering RNA (siRNA) targeting RhoA provided further evidence for RhoA’s role. Introducing Y27632 or RhoA siRNA under acidic conditions resulted in increased NPC migration, counteracting the effects on cytoskeletal organization and filopodia formation, and altering the differentiation patterns of NPCs. These findings collectively demonstrate that ASIC1a activation triggers the RhoA signaling pathway, negatively regulating migration and neurogenesis of NPCs under acidic conditions. This offers new insights into the molecular interplay between ASIC1a, RhoA, and cytoskeletal organization.
A Precise Target for Treatment
The study also indicated that blocking ASIC1a might specifically affect NPCs in the area of ischemic injury associated with acidosis without impacting those in normal brain areas, suggesting precise manipulation. The presence of functional ASICs in NPCs was confirmed by electrophysiological characterization, strengthening the case for targeting ASIC1a as a therapeutic strategy. The researchers concluded that since NPCs are the primary regenerative cells after CNS injury, exploring the effect of ASIC1a on NPCs and unraveling the relevant mechanism might fill a critical gap and provide a therapeutic target after ischemic injury.
The Need for Further Exploration
While this study provides compelling evidence for the role of ASIC1a in ischemic stroke recovery, further studies are needed, especially on the effect of ASIC1a inhibition on patients with cerebral infarction.
Targeting ASIC1a presents an interesting approach to promoting NPC migration and neurogenesis following ischemic stroke. This study’s findings shed light on previously unexplored interactions and offer a promising avenue for treatment of ischemic injury. As we continue to seek ways to mitigate the devastating impact of stroke, understanding and targeting ASIC1a could be a crucial step toward more effective therapies.
Antibodies
- Anti-ASIC1 Antibody (#ASC-014)
- Guinea pig Anti-ASIC1 Antibody (#ASC-014-GP) is raised in guinea pig and can be used in multiplex staining studies in conjunction with any of our antibodies raised in rabbit. This antibody has been raised against the same epitope as #ASC-014.
- Anti-ASIC2a Antibody (#ASC-012)
Pharmacological Tools
Blockers/Antagonists: peptides/peptide toxins
Activators/Agonists: peptides/peptide toxins
Activators/Agonists: small molecules
Modulators: peptides/peptide toxins
Explorer Kits

