Neurotrophins have emerged more than 6 decades ago as the mysterious molecules behind neuronal well-being. Their wide range of activities includes, but is not limited to, protection, proliferation and maintenance of all nerve cells. This short article presents a list papers citing the use of Alomone Labs NT-3 and NT-4 related products.
Introduction
Studies on neurotrophins (NTs) have tremendously contributed to our understanding of the underlying mechanisms of axonal viability, growth and development ever since research on nerve growth factor (NGF) over 60 years ago7. Together with NGF, the additional three vertebrate NTs, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4), are secreted from innervated, epithelium, fibroblast and immune-system cells5, in the peripheral or central nervous system (PNS or CNS) to generate various effects on neighboring neurons via different, yet occasionally entwined routes21. Specifically, both NT-3 and NT-4 standout due to their distinctive roles in maturation, proliferation and modulation of PNS and CNS synapses3-4,19, and are the topic of this short report.
All NTs are noncovalently-linked homodimers initially transcribed as 30-35 kDa precursors, with limited activity18. They are then cleaved by prohormone convertases to yield mature, active proteins (12-14 kDa). The four NTs share distinct structural homology, characterized by a cysteine knot motif and two pairs of antiparallel β strands. They also all contain a signal peptide and a pro-region with an N-linked glycolysation site3,19.
Three mammalian members of the tropomyosin-related kinase (Trk) receptors2 bind NTs with varied affinities: both BDNF and NT-4 activate TrkB, while NGF and NT-3 activate TrkA and TrkC, respectively; NT-3 also activates the other two Trks, albeit more weakly. Following ligand binding, Trks form dimers, and the subsequent kinase activation results in transphosphorylation of tyrosine residues which in turn enable the binding of cytoplasmic proteins that exhibit phosphotyrosine-binding (PTB) or Src-homology-2 (SH2) domains such as phospholipase C (PLC-γ1), p85 and Shc; interlaced kinase cascades involving RAS-mitogen-activated protein (Ras-MAP) or phosphatidyl inositol-3 (PI3), to name a couple, are then activated. Furthermore, a second receptor of the tumor necrosis factor superfamily, p75 (p75NTR), binds all NTs equally. p75NTR consists of a cysteine rich extracellular domain, a transmembrane domain and a cytoplasmic sequence notably known for executing programmed cell death3-4,19.
Hippocampus
The renowned abundance of all four NTs in the hippocampus has stimulated a research of their different spatiotemporal secretion patterns. As was previously shown, NTs elicit hippocampal long-term potentiation (or long-term depression; LTP or LTD) via activation of TrkB by means of enhanced excitatory (or inhibitory) input. However, a degree of obscurity masks the NTs’ relative contribution to the observed synaptic modifications1,21-22. Just how are the hippocampal NTs balanced, in regard to constitutive and regulatory pathways, has begun to be unmasked. Recombinant human beta-NGF protein (#N-245), Recombinant human BDNF protein (#B-250), and Recombinant human Neurotrophin-4 (NT-4) protein (#N-270) were added to PC12 cells (which natively express TrkA), cotransfected with TrkB and GFP. Procedural differentiation to chromaffin neurons was then achieved by applying NGF, in order to reliably model hippocampi neurons. All NTs were expressed similarly in both the differentiated and non-differentiated cells and localized distally in the former, and proximally in the latter, a result which coincides with previous observations in rat hippocampus, where both NT-4 and NGF were targeted to the regulatory pathway (corresponding to the distal locale) in 23-26% of the cells whereas NT-3 and BDNF expressed almost exclusively in the constitutive pathway (corresponding to the proximal locale). Together, these data collectively suggest that NTs’ localization is cell specific6.
It is noteworthy to mention, that PC12 cells can also acquire neuronal phenotypes when cultured in preconditioned media from Schwann cells consisting of Recombinant human beta-NGF protein, Recombinant human BDNF protein, Recombinant human Neurotrophin-4 (NT-4) protein but lacking Recombinant human Neutrotrophin-3 (NT-3) protein (#N-260). Not surprisingly, NGF alone was able to induce neural outgrowth of PC12 cells1. Poly(ADP-ribose) polymerase-1 (PARP-1), a protein activated in response to DNA-damaging agents was found to be independently activated following exposure to Alomone Labs NGF and other neurotrophins like Recombinant human BDNF protein and Recombinant human Neurotrophin-3 (NT-3) protein of rat cortical neurons (Figure 1). PARP-1 was also activated in the neurite outgrowth of PC12 cells induced by NGF exposure22.
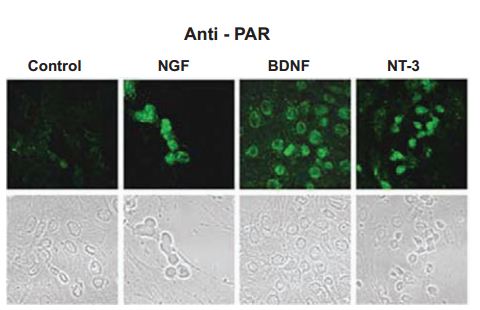
Immunocytochemical staining of rat cortical neurons treated with Alomone Labs NGF, Recombinant human BDNF protein (#B-250) and Recombinant human Neurotrophin-3 (NT-3) protein (#N-260) neurotrophic factors (100 ng/ml). Following treatment, cells were labeled with anti-par.
Adapted from reference 22 with permission of The Society for Neuroscience.
Further understanding of NT secretion particularities has been gained in a study utilizing the percolation approach (a method which enables the global, statistical measurement of the average number of input connections per neuron)16, in which rat hippocampal neuronal-circuitry connectivity was evaluated. Alomone Labs Recombinant human BDNF protein and Recombinant human Neurotrophin-3 (NT-3) protein excited their target network beyond a defined threshold, thereby forming connected clusters which enabled spontaneous bursting of the whole framework. Matching electrophysiological recordings showed that EPSCs increased by 125% upon NT treatment. This enhanced connectivity resulted from an increased number of input connections rather than from manifestation of the synaptic strength16. In addition, neurons developed connectivity earlier when treated with Recombinant human BDNF protein and Recombinant human Neurotrophin-3 (NT-3) protein when compared to control conditions. Interestingly, in a kindling rat model NT-3 inflicted modulatory effects on both BDNF and its immature form – proBDNF. Increased BDNF levels following kindling were decreased after human NT-3 administration. On the other hand, proBDNF levels which did not change in kindled animals increased after human NT-3 was administered. The reciprocal effect of BDNF on NT-3 was not observed, such that NT-3 levels remained constant in western blot analysis using Anti-Neurotrophin 3 (NT-3) Antibody (#ANT-003), following BDNF treatment21.
Cortex
In the developing brain, NT-3 has also been found to have great importance. In an attempt to isolate the environmental factors which assist the maturation of cortical progenitors, Ohtsuka et al. injected human NT-3 into the embryos of pregnant mice, whose cortical progenitors were labeled with 5-bromo-2’-deoxyuridine (BrdU) – commonly used to detect proliferating cells. NT-3 promoted cell proliferation by ~41.5%, without diverting their location17. Cellular proliferation mediated by NT3 required the phosphorylation/activation of EKR1/2 and ERK5 kinases. ERK1/2 was also examined in a study investigating the role of its signaling cascade in switching the NMDA receptor subunit composition from NR2B to NR2C in the developing cerebellum. BDNF in cerebellar granule cells treated with low amount (5 nM) of KCl up-regulated NR2C mRNA – an effect which was silenced upon application of either ERK1/2 or ERK5 inhibitors, suggesting that their phosphorylation is essential for NR2C mRNA upregulation. NT-4 and NT-3 (both purchased from Alomone Labs) treatment had similar effects20.
Cochlea (and Olfactory)
The cochlear nucleus (CN) of the auditory system provides a useful means to study the developmental effects of NTs due to its subdivision into more than a dozen distinct regions, each containing a limited number of unique cell types. Using Anti-Neurotrophin 3 (NT-3) Antibody in immunohistochemical staining, NT-3 was detected in cochlear axons around the soma of ventral CN axons at postnatal day 30 (P30) suggesting an anterograde transportation of NT-3 into the CN9. The same team also demonstrated that NT-3 is detected in precursor cells at the time of their migration, but disappears upon birth, while TrkC’s expression time-span encompasses that of its ligand appearance14. In their previous effort, they employed the same antibodies to conclude that in the cochlear ganglion (CG), fibroblast growth factor 2 (FGF2), a recognized mitogen in the nervous system, enhances proliferation, migration and differentiation of pre-terminal axons upon interaction with NT-314-15.
The importance of NT-3 is also evident in the operating room; for instance, it has been used to grow purified olfactory ensheathing cells from nasal biopsy origin, which were later successfully transplanted to the injured spinal cord of paraplegic patients in a novel procedure, proved efficacious 12 months post-surgery10.
Neuromuscular Junctions (NMJs)
Formation of proper innervation at the neuromuscular junction (NMJ) is highly dependent on the timed release of acetylcholine (ACh), a process affected by key NTs during synaptogenesis. Immunohistochemistry using Anti-Neurotrophin 3 (NT-3) Antibody carried out by Garcia et al. confirmed the presence of exogenously applied Recombinant human Neurotrophin-3 (NT-3) protein (from Alomone Labs) within the synaptic elements of the NMJ; the group analyzed a set of experiments which yielded that at postnatal day 6 (P6), exogenous NT-3 enhances the end plate potentials (EPPs) of competitive immature nerve endings when the endplates are polyinnervated. In adulthood, it fails to confer any effect12, whereas exogenous addition of Alomone Labs Recombinant human Neurotrophin-4 (NT-4) protein in adult NMJs potentiates ACh release9. BDNF, on the other hand, enhances ACh release when binding to p75NTR and inhibits ACh release upon binding to TrkB11. Further, the same authors showed that the reciprocal function of TrkB and muscarinic-ACh receptors (mAChR) is necessary for the coupling of TrkB to ACh13.
NT-3 and NTs are frequently being used as supplementary factors in the growing media of neuronal cultures. For example, their presence is vital for the proliferation of motoneurons dependent on the release of nitric oxide (NO) from ventral horn interneurons23. Also, they are important for delineating the phenotypic traits of the masseter muscle afferents, which innervate the jaw8.
Alomone Labs is pleased to offer Recombinant human Neurotrophin-3 (NT-3) protein (#N-260), Recombinant human Neurotrophin-4 (NT-4) protein (#N-270) as a well as antibodies targeted to them; Anti-Neurotrophin 3 (NT-3) Antibody (#ANT-003), Anti-proNT-3 Antibody (#ANT-012) which recognizes only the “pro” form of NT3 and Anti-Neurotrophin 4 (NT-4) Antibody (#ANT-004). Antibodies against their receptors TrkB, TrkC and p75NTR are also available.
References
- Bampton, E.T. and Taylor, J.S. (2005) J. Neurobiol. 63, 29.
- Benito-Gutierrez, E. et al. (2005) Development 132, 2191.
- Berry, A. et al. (2012) Neural Plast. 2012, 784040.
- Binder, D.K. and Scharfman, H.E. (2004) Growth Factors 22, 123.
- Braun, A. et al. (1999) Am. J. Respir. Cell. Mol. Biol. 21, 537.
- Brigadski, T. et al. (2005) J. Neurosci. 25, 7601.
- Cohen, S. et al. (1954) Proc. Natl. Acad. Sci. U.S.A. 40, 1014.
- Connor, M. et al. (2005) Mol. Pain 1, 31.
- Feng, J. et al. (2010) J. Neurosci. Res. 88, 86.
- Feron, F. et al. (2005) Brain 128, 2951.
- Garcia, N. et al. (2010) J. Neurosci. Res. 88, 1406.
- Garcia, N. et al. (2010) Neurosci. Lett. 473, 141.
- Garcia, N. et al. (2010) J. Neurosci. 30, 16514.
- Hossain, W.A. et al. (2006) J. Neurobiol. 66, 897.
- Hossain, W.A. et al. (2008) J. Neurosci. Res. 86, 2376.
- Jacobi, S. et al. (2009) Eur. J. Neurosci. 30, 998.
- Ohtsuka, M. et al. (2009) J. Neurosci. Res. 87, 301.
- Pang, P.T. et al. (2004) Science 306, 487.
- Reichardt, L.F. (2006) Philos. Trans. R. Soc. Lond. B. Biol. Sci. 361, 1545.
- Suzuki, K. et al. (2005) J. Neurosci. 25, 9535.
- Ullal, G.R. et al. (2007) Neurochem. Int. 50, 866.
- Visochek, L. et al. (2005) J. Neurosci. 25, 7420.
- Xiong, G. et al. (2007) Eur. J. Neurosci. 25, 1987.