Overview
- Peptide (C)RHHRHRDRDKTSASTPA, corresponding to amino acid residues 851-867 of rat CACNA1B (Accession Q02294). Intracellular loop between domains II and III.

- Transfected tsA201 cells (1:200) (Marangoudakis, S. et al. (2012) J. Neurosci. 32, 10365.).
 Expression of CaV2.2 in mouse cerebellumImmunohistochemical staining of mouse cerebellum with Anti-CACNA1B (CaV2.2) Antibody (#ACC-002), (1:100). A. CaV2.2 (red) appears in Purkinje cells (arrows) and is distributed diffusely in the molecular layer (Mol). B. Staining of Purkinje cells with mouse anti-Calbindin 28K (green) demonstrates the restriction of CaV2.2 to cell bodies but not to dendrites in the molecular layer. C. Merged image of panels A and B.
Expression of CaV2.2 in mouse cerebellumImmunohistochemical staining of mouse cerebellum with Anti-CACNA1B (CaV2.2) Antibody (#ACC-002), (1:100). A. CaV2.2 (red) appears in Purkinje cells (arrows) and is distributed diffusely in the molecular layer (Mol). B. Staining of Purkinje cells with mouse anti-Calbindin 28K (green) demonstrates the restriction of CaV2.2 to cell bodies but not to dendrites in the molecular layer. C. Merged image of panels A and B.
 Expression of CaV2.2 in rat DRG primary cultureImmunocytochemical staining of paraformaldehyde-fixed and permeabilized rat dorsal root ganglion (DRG) primary culture. A. Cells were stained using Anti-CACNA1B (CaV2.2) Antibody (#ACC-002), (1:200) followed by goat anti-rabbit-AlexaFluor-555 secondary antibody. B. Nuclear fluorescence staining of cells using the membrane-permeable DNA dye Hoechst 33342. C. Merged images of panels A and B.
Expression of CaV2.2 in rat DRG primary cultureImmunocytochemical staining of paraformaldehyde-fixed and permeabilized rat dorsal root ganglion (DRG) primary culture. A. Cells were stained using Anti-CACNA1B (CaV2.2) Antibody (#ACC-002), (1:200) followed by goat anti-rabbit-AlexaFluor-555 secondary antibody. B. Nuclear fluorescence staining of cells using the membrane-permeable DNA dye Hoechst 33342. C. Merged images of panels A and B.- NCI-H295R human adrenocortical cell line (H295R) (1:100) (Aritomi, S. et al. (2011) Hypertens. Res. 34, 193.).
- Tsunemi, T. et al. (2002) J. Biol. Chem. 277, 7214.
- Beuckmann, C.T. et al. (2003) J. Neurosci. 23, 6793.
- Scheuber, A. et al. (2004) J. Neurosci. 24, 10402.
- Dascal, N. (2001) Trends Endocrinol. Metab. 12, 391.
- McCleskey, E.W. et al. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 4327.
- Feng, Z.P. et al. (2001) J. Biol. Chem. 276, 15728.
Voltage-dependent Ca2+ channels (CaV channels) are pivotal players in many physiological roles such as secretion, contraction, migration and excitation.1
The voltage-dependent Ca2+ channels are composed of several subunits; α1, β, α2δ and γ. CaV channels were originally divided into six physiological types: L-, N-, P-, Q-, R-, and T-type.
The CaV2.2 (formally named α1B) composes the α1 poreforming subunit for the N-type Ca2+ channel family. They are involved in neurotransmitter release from central neurons, including glutamate, γ-aminobutyric acid, acetylcholine, dopamine and noradrenaline.2
The CaV2.2 is expressed preferentially in the central nervous system, where along with CaV2.1, it is responsible for pre-synaptic Ca2+ influx and neurotransmitter release.1,3
The CaV2.2 channel is negatively regulated by many different GPCRs. There are two ways that this is done: either by directly binding Gβγ to the channel or by an indirect mechanism involving second messenger and channel phosphorylation.4
ω-Conotoxin GVIA (#C-300) is a specific blocker of CaV2.2 Ca2+ channels. It specifically blocks N-type CaV channels by binding to the CaV2.2 α1 subunit (α1B) and its action is only partially reversible.5,6
Application key:
Species reactivity key:
Anti-CACNA1B (CaV2.2) Antibody (#ACC-002) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot, immunoprecipitation, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize CaV2.2 from mouse, rat, and human samples.
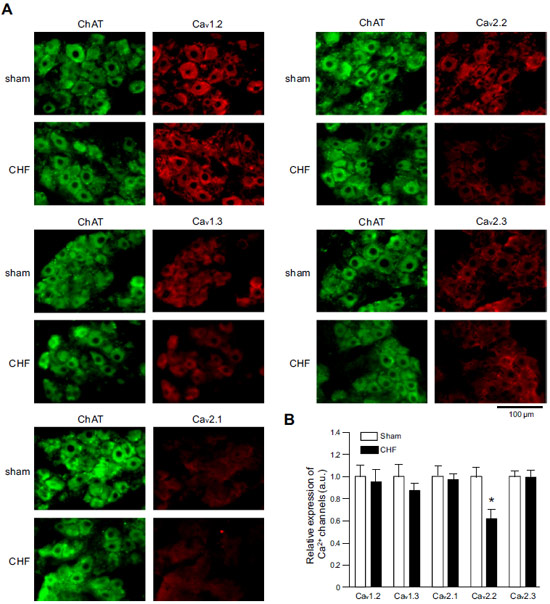 Expression of CaV α subunits in rat ICG.Immunohistochemical staining of rat intracardiac ganglia (ICG) using Anti-CaV1.2 (CACNA1C) Antibody (#ACC-003), Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005), Anti-CACNA1A (CaV2.1) Antibody (#ACC-001), Anti-CACNA1B (CaV2.2) Antibody (#ACC-002) and Anti-CaV2.3 (CACNA1E) Antibody (#ACC-006). All CaV subtypes are expressed in sham treated rats but N-type CaV channel levels are decreased in CHF rats.Adapted from Tu, H. et al. (2014) with permission of the American Physiological Society.
Expression of CaV α subunits in rat ICG.Immunohistochemical staining of rat intracardiac ganglia (ICG) using Anti-CaV1.2 (CACNA1C) Antibody (#ACC-003), Anti-CaV1.3 (CACNA1D) Antibody (#ACC-005), Anti-CACNA1A (CaV2.1) Antibody (#ACC-001), Anti-CACNA1B (CaV2.2) Antibody (#ACC-002) and Anti-CaV2.3 (CACNA1E) Antibody (#ACC-006). All CaV subtypes are expressed in sham treated rats but N-type CaV channel levels are decreased in CHF rats.Adapted from Tu, H. et al. (2014) with permission of the American Physiological Society.Applications
Citations
- Western blot and immunohistochemistry of mouse adrenal gland lysates and sections, respectively. Tested in CaV2.2-/- mice.
Murakami, M. et al. (2007) Biochem. Biophys. Res. Commun. 354, 1016.
- Mouse calyx of Held lysate.
Sun, Z.C. et al. (2016) Sci. Rep. 6, 21774. - Rat primary submucosa cells.
Rehn, M. et al. (2013) Cell Tissue Res. 353, 355. - Mouse synaptosome (1:300).
Saggu, S. et al. (2013) Schizophr. Res. 146, 254. - Mouse adrenal gland membrane lysates. Also tested in CaV2.2-/- mice.
Murakami, M. et al. (2007) Biochem. Biophys. Res. Commun. 354, 1016.
- Transfected tsA201 cells (1:200).
Marangoudakis, S. et al. (2012) J. Neurosci. 32, 10365.
- Mouse kidney sections.
Ohno, S. et al. (2016) Sci. Rep. 6, 27192. - Rat retinal sections (1:1000).
Sargoy, A. et al. (2014) PLoS ONE 9, e84507. - Rat intracardiac ganglia and stellate ganglia neurons.
Tu, H. et al. (2014) Am. J. Physiol. 306, C132. - Rat retinal sections (1:1000-1:1500).
Liu, X. et al. (2013) J. Physiol. 591, 3309. - Mouse adrenal gland sections. Also tested in CaV2.2-/- mice.
Murakami, M. et al. (2007) Biochem. Biophys. Res. Commun. 354, 1016.
- Rat adipose-derived stromal cells and rat bone marrow stromal cells.
Forostyak, O. et al. (2016) Stem Cell Res. 16, 622. - Rat stellate neurons (1:200).
Larsen, H.E. et al. (2016) J. Neurosci. 36, 8562. - Rat primary submucosa cells (1:100).
Rehn, M. et al. (2013) Cell Tissue Res. 353, 355. - NCI-H295R human adrenocortical cell line (H295R) (1:100).
Aritomi, S. et al. (2011) Hypertens. Res. 34, 193.
- Mishima, K. et al. (2013) Am. J. Physiol. 304, F665.
- Lv, P. et al. (2012) J. Neurosci. 32, 16314.
- Shoham, S. et al. (2001) Biol. Psychiatry 49, 876.
- Schiff, M.L. et al. (2000) Nature 408, 723.
- Singer-Lahat, D. et al. (2000) Eur. J. Physiol. 440, 627.
- Bae, I.H. et al. (1999) Korean J. Biol. Sci. 3, 53.
- Barbosa, J. et al. (1999) J. Neurochem. 73, 1881.
- Lopez, I. et al. (1999) Neuroscience 92, 773.
- Macleod, G.T. et al. (1999) J. Neurophysiol. 82, 1133.
- Serrano, C.J. et al. (1999) FEBS Letters 462, 171.