Overview
- Horne, A.L. et al. (1992) Br. J. Pharmacol. 107, 732.
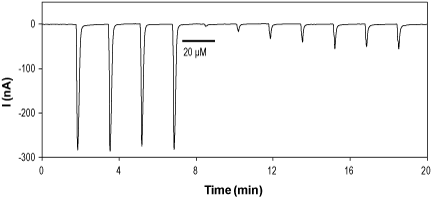 Alomone Labs (+)-Bicuculline inhibits GABA(A) α2/β1/γ2 currents activated by γ-Aminobutyric acid in heterologously expressing Xenopus oocytes.Time course of γ-Aminobutyric acid (#G-110) (100 µM) activated GABA(A) α2/β1/γ2 currents and their inhibition by 20 µM (+)-Bicuculline (#B-135), which was perfused for 100 seconds as indicated by the horizontal bar. Holding membrane potential was -60 mV and γ-aminobutyric acid was applied every 100 seconds.
Alomone Labs (+)-Bicuculline inhibits GABA(A) α2/β1/γ2 currents activated by γ-Aminobutyric acid in heterologously expressing Xenopus oocytes.Time course of γ-Aminobutyric acid (#G-110) (100 µM) activated GABA(A) α2/β1/γ2 currents and their inhibition by 20 µM (+)-Bicuculline (#B-135), which was perfused for 100 seconds as indicated by the horizontal bar. Holding membrane potential was -60 mV and γ-aminobutyric acid was applied every 100 seconds.
- Briggs, S.W. and Galanopoulou, A.S. (2011) Neural Plast. 2011, 527605.
- Khakhalin, A.S. (2011) J. Neurophysiol. 106, 1065.
- Esmaeili, A. et al. (2009) J. Neurophysiol. 101, 341.
- Ueno, S. et al. (1997) J. Neurosci. 17, 625.
- Walker, J. et al. (2012) PLoS One 7, e31415.
- Paine, T.A. et al. (2011) Neuropsychopharmacology 36, 1703.
γ-Aminobutyric acid (GABA) is an amino acid derivate, synthesized from glutamate by the pyridoxal-L-phosphate (PLP)-dependent enzyme, glutamate decarboxylase (GAD)1. GABA provides the primary inhibitory signaling in the adult mammalian brain (CNS), and, to some degree of controversy, exerts a depolarizing response in the developing brain2.
GABA is an agonist of two main receptor classes in the CNS: GABAA, an ionic ligand-gated channel, and GABAB, a metabotrophic, G-protein coupled receptor. GABAA is assembled by 5 out of 16 possible subunits with α, β and either a γ or a δ (2:2:1 stoichiometry) being the most common. With an EC50 between 3 to 12 μM it interacts with GABA to allow either an influx or an efflux of Cl- ions into and out of the cell, respectively3.
Bicuculline (BIC) is a competitive allosteric inhibitor of GABAA-induced currents with an IC50 of 1-3 μM4. BIC has been employed to model epileptic seizures in hippocampal slices5, to induce schizophrenia-like symptoms in the pre-frontal cortex6, and is an essential research tool in the study of various psychomotor and behavioral defects4-6.
(+)-Bicuculline (#B-135) is a highly pure, synthetic, and biologically active compound.
Applications
Citations
- Tang, Y. et al. (2018) Eur. J. Neurosci. 47, 866.

