Overview
- Bocchini, V. and Angeletti, P.U. (1969) Proc. Natl. Acad. Sci. U.S.A. 64, 787.
- Huang, E.J. and Reichardt, L.F. (2001) Annu. Rev. Neurosci. 24, 677.
- Teng, K.K. and Hempstead, B.L. (2004) Cell Mol. Life Sci. 61, 35.
 Alomone Labs Native mouse NGF 2.5S protein (>95%) promotes neurite outgrowth and survival in PC12 cells.Upper panel: PC12 cells were grown in absence of serum and in the presence of varying concentrations of Native mouse NGF 2.5S protein (>95%) (#N-100), the cell survival after 24 h was measured by XTT method and calculated as a relative percentage of the control and plotted against concentrations. Lower panel: PC12 cells were grown on collagen-coated plates in absence (1) or presence (2) of 4 nM Native mouse NGF 2.5S protein (>95%). The development of neurites over a period of 6 days was visualized by Methylene Blue staining.
Alomone Labs Native mouse NGF 2.5S protein (>95%) promotes neurite outgrowth and survival in PC12 cells.Upper panel: PC12 cells were grown in absence of serum and in the presence of varying concentrations of Native mouse NGF 2.5S protein (>95%) (#N-100), the cell survival after 24 h was measured by XTT method and calculated as a relative percentage of the control and plotted against concentrations. Lower panel: PC12 cells were grown on collagen-coated plates in absence (1) or presence (2) of 4 nM Native mouse NGF 2.5S protein (>95%). The development of neurites over a period of 6 days was visualized by Methylene Blue staining.
- Roux, P.P. and Barker P.A. (2002) Prog. Neurobiol. 67, 203.
- Levi-Montalcini, R. (1966) Harvey Lect. 60, 217.
- Farinas, I. et al. (1998) Neuron 21, 325.
- Levi-Montalcini, R. et al. (1996) Trends Neurosci. 19, 514.
- Bradshaw, R.A. (1978) Annu. Rev. Biochem. 47, 191.
- Bocchini, V. and Angeletti, P.U. (1969) Proc. Natl. Acad. Sci. U.S.A. 64, 787.
- McDonald, N.Q. et al. (1991) Nature 354, 411.
- Huang, E.J. and Reichardt, L.F. (2001) Annu. Rev. Neurosci. 24, 677.
- Freund V. and Frossard, N. (1994) Prog. Brain Res. 146, 335.
- Raychaudhuri, S.P. and Raychaudhuri, S.K. (2004) Prog. Brain Res. 146, 433.
- Kawamoto, K. and Matsuda, H. (2004) Prog. Brain Res. 146, 369.
- Teng, K.K. and Hempstead, B.L. (2004) Cell Mol. Life Sci. 61, 35.
- Darling, T. and Shooter, E.M. (1984) "Methods for preparation and assay of nerve growth factor", Cell Culture Methods for Molecular and Cellular Biology, vol. 4 (Eds. D. W. Barnes, D.A. Sinbasku, and G.H. Sato), pp. 79-83, Alan R. Liss, New York.
The neurotrophins ("neuro" means nerve and "trophe" means nutrient) are a family of soluble, basic growth factors which regulate neuronal development, maintenance, survival and death in the CNS and PNS.1
NGF, the first member of the family to be discovered, was originally purified as a factor able to support survival of sympathetic and sensory spinal neurons in culture.2 It is synthesized and secreted by sympathetic and sensory target organs and provides trophic support to neurons as they reach their final target.3
Neurotrophin secretion increases in the nervous system following injury. Schwann cells, fibroblasts, and activated mast cells normally synthesize NGF constitutively, however direct trauma and induction of cytokines combine to increase neurotrophin production in these cells after injury.4
NGF is purified in three forms: the 7S, 2.5S and β. The 7S (130 kDa) form occurs naturally in mouse submaxillary glands, and is a multimeric protein composed of two α, one β and two γ subunits. The name is derived from its sedimentation co-efficient, 7S.
The biologically active subunit is the β, which is a 26 kDa dimer composed of two identical 120 amino acid chains held together by hydrophobic interactions.5 The 2.5S form is 9 amino acids shorter than the β form because of proteolysis that occurs during the purification process.6 The structural hallmark of all the neurotrophins is the characteristic arrangement of the disulfide bridges known as the cysteine knot, which has been found in other growth factors such as Platelet-derived growth factor.7 There is a 95.8% homology between the rat and mouse forms, and a 85% homology between the human and mouse.
NGF has been shown to regulate neuronal survival, development, function and plasticity.8 Recently, involvement of NGF in processes not involving neuronal cells has been shown, such as asthma,9 psoriasis10 and wound healing.11 The biological effects of NGF are mediated by two receptors: TrkA, which is specific for NGF, and p75NTR, which binds all the neurotrophins.12
Native mouse NGF 2.5S protein (>95%) (#N-100) is a highly pure, natural, and biologically active protein.
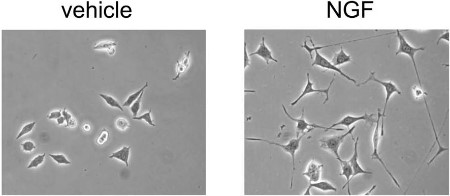
Alomone Labs Native mouse NGF 2.5S protein induces neurite outgrowth in PC12 cells.Neurite outgrowth in PC12 cells was induced by the addition of 50 ng/ml Native mouse NGF 2.5S protein (>95%) (#N-100) or Native mouse NGF 2.5S protein (99%) (#N-240) for 24 hrs.Adapted from Terada, K. et al. (2014) PLoS ONE 9, e93223. with permission of PLoS.
Applications
Citations
- Usuki, S. et al. (2019) Biochem. Biophys. Rep. 17, 132.
- Namer, B. et al. (2017) Front. Neurol. 8, 335.
- Oddone, F. et al. (2017) PLoS ONE 12, e0168565.
- Rohr, D. et al. (2017) Glia 65, 1186.
- Takahashi, N. et al. (2017) FEBS OPEN Bio 7, 1338.
- Wang, L.C. et al. (2017) J. Neurochem. 140, 451.
- Nakajima, K. and Marunaka, Y. (2016) Biochem. Biophys. Res. Commun. 479, 338.
- Wang, L.C. and Almazan, G. (2016) Glia 64, 1021.
- An, J.M. et al. (2015) Eur. J. Pharmacol. 748, 37.
- Calvo, L. et al. (2015) J. Neurosci. 35, 7190.
- Fischer, M.J. et al. (2015) PLoS ONE 10, e0123762.
- Yu, L. et al. (2015) Front. Mol. Neurosci. 8, 2.
- De Paula, M.L. et al. (2014) Glia 62, 64.
- Malsch, P. et al. (2014) J. Neurosci. 34, 9845.
- Mnich, K. et al. (2014) Cell Death Dis. 5, e1202.
- Quarta, S. et al. (2014) J. Neurosci. 34, 13222.
- Ronzitti, G. et al. (2014) J. Neurosci. 34, 10603.
- Terada, K. et al. (2014) PLoS ONE 9, e93223.
- Walker, C.S. et al. (2014) Br. J. Pharmacol. 171, 1521.
- Cardinale, A. et al. (2012) J. Biol. Chem. 287, 2618.
- Green, R.A. et al. (2012) Biomaterials 33, 5875.
- Mohamed, A. et al. (2012) J. Neurosci. 32, 6490.
- Sirabella, R. et al. (2012) J. Neurochem. 122, 911.
- Velanac, V. et al. (2012) Glia 60, 203.
- Bucci, G. et al. (2011) J. Physiol. 589, 3085.
- Haeusgen, W. et al. (2011) Cell. Signal. 23, 1281.
- Nakajima, K. et al. (2011) Cell Physiol. Biochem. 28, 147.
- Fischer, M.J. et al. (2010) J. Biol. Chem. 285, 34781.
- Schoenmann, Z. et al. (2010) J. Neurosci. 30, 6375.
- Yamamoto, Y. et al. (2009) J. Biol. Chem. 284, 12480.
- Yao, L. et al. (2009) Acta Biomater. 5, 580.
- Leffler, A. et al. (2008) J. Clin. Invest. 118, 763.
- Madziar, B. et al. (2008) J. Neurochem. 107, 1284.
- Marler, K.J. et al. (2008) J. Neurosci. 28, 12700.
- Yung, L.Y. et al. (2008) Cell. Signal. 20, 1538.
- Arthur, D.B. et al. (2007) J. Neurochem. 100, 1257.
- Carter, J.M. et al. (2007) J. Biol. Chem. 283, 202.
- Fragoso, G. et al. (2007) Glia 55, 15531.
- Fuenzalida, K. et al. (2007) J. Biol. Chem. 282, 37006.
- Ma, H. and Mochida, S. (2007) Neurosci. Res. 57, 491.
- Saavedra, L. et al. (2007) J. Biol. Chem. 282, 35722.
- Zhang, Z. et al. (2007) J. Neurosci. 27, 2693.
- Docherty, R.J. and Farrag, K.J. (2006) Neuropharmacology 51, 1047.
- Song, M.S. et al. (2006) Neurobiol. Aging 27, 1224.
- Wise, H. (2006) Eur. J. Pharmacol. 535, 69.
- Kanjhan, R. et al. (2005) J. Pharmacol. Exp. Ther. 314, 1353.
- O’Driscoll, C.M. and Gorman, A.M. (2005) Neuroscience 131, 321.
- Zarain-Herzberg, A. et al. (2005) Mol. Brain. Res. 137, 235.
- Kobayashi, H. et al. (2004) Neuroscience 125, 973.
- Lutz, M. et al. (2004) FEBS J. 271, 920.
- Nishio, M. et al. (2004) J. Biol. Chem. 279, 33368.
- Sato, S. et al. (2004) Gene 329, 51.
- Darbon, P. et al. (2003) J. Neurophysiol. 90, 3119.
- Hewett, J. et al. (2003) J. Neurosci. Res. 72, 158.
- Katzir, I. et al. (2003) Toxicon 42, 481.
- Lambeng, N. et al. (2003) Mol. Brain Res. 111, 52.
- Waetzig, V. and Herdegen, T. (2003) J. Biol. Chem. 278, 567.
- Avelino, A. et al. (2002) Brain. Res. 951, 264.
- Brann, A.B. et al. (2002) J. Biol. Chem. 277, 9812.
- Lambeng, N. et al. (2001) Neurosci. Lett. 297, 133.
- Meilander, N.J. et al. (2001) J. Control. Release 71, 141.
- de Chaves, E.P. et al. (2001) J. Biol. Chem. 276, 36207.
- Shepherd, S.P. and Holzwarth, M.A. (2001) Am. J. Physiol. 280, C61.
- Vanhoutte, P. et al. (2001) J. Biol. Chem. 276, 5189.
- Cordeiro, M.L. et al. (2000) J. Neurochem. 75, 2622.
- Gil, C. et al. (2000) FEBS Lett. 481, 177.
- Posse De Chaves, E. et al. (2000) J. Biol. Chem. 275, 19883.
- Rende, M. et al. (2000) Int. J. Dev. Neurosci. 18, 869.
- Coulson, E.J. et al. (1999) J. Biol. Chem. 274, 16387.
- Lambeng, N. et al. (1999) Brain Res. 821, 60.
- Moore, K.A. et al. (1999) J. Physiol. 514, 111.
- Bennett, G. et al. (1998) Pain 77, 315.
- Thippeswamy, T. and Morris, R. (1997) Neurosci. Lett. 230, 9.
