The importance of the Aquaporin water channels was underscored by awarding the 2003 Nobel Prize in Chemistry to Peter Agre “for the discovery of water channels”. Many scientific articles have been published describing the Aquaporins’ structure, function and tissue distribution. Among the many methods that were used and contributed to the enormous progress in the field, antibodies played an important role. This article describes the use of Alomone Labs antibodies serving as a powerful tool in the ongoing research.
Introduction
Water is a major component of the cell, forming between 70 and 95% of its mass. It can cross lipid bilayers of all biological membranes by simple diffusion. However, diffusion is a slow, non-regulated process which cannot account for selective transmembrane water permeation as occurs in many fluid transporting tissues during normal physiological processes. This suggested the existence of additional pathways for water flux. The recent discovery of water channels, Aquaporins (AQPs), provides a molecular explanation for the rapid and regulated transport of water through lipid bilayers1.
The Aquaporin family consists of 13, small and hydrophobic proteins named AQP0 to AQP121,24 . The water channels share six transmembrane domains with an intracellular N- and C-termini structure but differ in their tissue distribution as well as in their regulatory mechanism. The functional channel is a tetramer comprised of four functionally independent pores. They are expressed in a subset of epithelia that have 10 to 100-fold higher capacities for water permeation such as kidney tubules and red blood cells1.These channels facilitate osmotically driven water transport and are divided into two groups based on their permeability characteristics: AQP1, AQP2, AQP4, AQP5 and AQP8 are considered primarily as water selective channels. AQP3, AQP7, AQP9 and AQP10 transport water as well as glycerol and are also called Aquaglyceroporins24.
Aquaporins in the Kidney
Water re-absorption occurs in different regions of the kidney and involves several members of the Aquaporin family: AQP1, AQP2, AQP3, AQP4, AQP6 and AQP7.
An important role in maintaining water homeostasis was first attributed to Aquaporins. This discovery subsequently led to countless of studies directed to explore their role in kidney failure, injury or transplantation.
Aquaporin 1
Aquaporin 1 is selectively permeated by water driven by osmotic gradients. AQP1 is expressed in the apical and basolateral membranes of renal proximal tubules as well as in the descending thin limbs of Henle’s loop and the descending vasa recta endothelia1,24. Studies with mice lacking AQP1 show that the channel has a critical role in urine concentration13. Induction of renal dysfunction by injection of cisplatin in rats demonstrated urine concentration defects in association with decreased expression of AQP1, AQP2 and AQP3 as was demonstrated using Anti-Aquaporin 1 Antibody (#AQP-001), Anti-Aquaporin 2 Antibody (#AQP-002) and Anti-Aquaporin 3 Antibody (#AQP-003)8 (Figure 1). Co-treatment of rats with cisplatin and α-Lipoic acid prevented kidney dysfunction and restored AQP1-AQP3 expression as was demonstrated using all three Alomone Labs respective antibodies8,4,7.
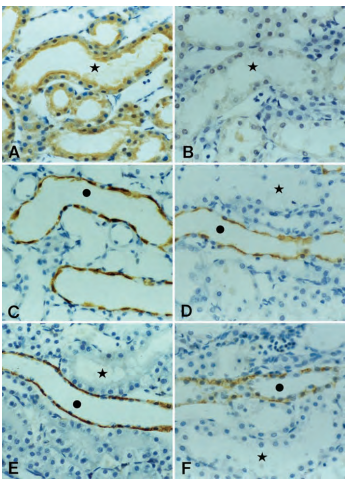
Immunohistochemical staining of AQP1, AQP2, and AQP3 in the outer medulla from control and cisplatin-treated rats using Anti-Aquaporin 1 Antibody (#AQP-001), Anti-Aquaporin 2 Antibody (#AQP-002) and Anti-Aquaporin 3 Antibody (#AQP-003). A) Immunoreactivity for AQP1 was most prominent in the apical membrane of proximal tubules. B) The AQP1 labeling decreased markedly with cisplatin treatment. C) The abundance of AQP2 labeling was shown exclusively in the collecting duct principal cells, both in the apical region and throughout the cytoplasm. D) The AQP2 labeling decreased by cisplatin. E) AQP3 was localized to the basolateral membrane of the collecting duct principal cells. F) The AQP3 labeling decreased in the outer medullary collecting duct in response to cisplatin. *, S3 segment of the proximal tubule; •, collecting duct. Magnification, X350.
Adapted from reference 8 with permission of the American Society of Nephrology.
Aquaporin 2 and Aquaporin 3
Aquaporin 2 is mainly expressed in the principal cells of renal collecting ducts. It is primarily abundant in intracellular vesicles and apical membranes1 . Regulation of AQP2 trafficking from intracellular vesicles to the cell surface appears to be a complex process that is regulated by vasopressin1,10,22.
Aquaporin 3 is expressed at the basolateral membrane of principal cells in the collecting ducts. Although it is a member of the Aquaporin family, its biophysical function is slightly different since it is also permeable to glycerol, and is therefore considered to be an Aquaglyceroporin channel1.
Anti-Aquaporin 2 Antibody was used in many works to demonstrate alteration in AQP2 expression following different treatments or in different pathological statuses. Analysis of kidneys from deoxycorticosterone acetate (Doca)-salt hypertensive rats demonstrated a significantly enhanced expression of AQP2 in the cortex and outer medulla compared to control groups as well as enhanced shuttling as was demonstrated by western blot analysis of cortex, outer medulla and inner medulla of DOCA-salt kidneys using Anti-Aquaporin 2 Antibody10. Increased expression of medullary AQP1, AQP2 and AQP3 was also demonstrated in spontaneously hypertensive rats using Anti-Aquaporin 2 Antibody and Anti Aquaporin 3 Antibody11. However, similar work with DOCA-salt hypertensive rats done by Bae et al. resulted with opposite results, demonstrating a decrease in AQP1-3 expression4. The discrepancies between the two experiments, might have been a result of methodological differences, Na+ and water balance differences and the different sensitivity to Na+ and water intake.
The decrease in AQP expression is associated in many cases with the obstruction of kidneys or kidney failure. Immunohistochemical staining of obstructive kidneys compared to controls demonstrated a decrease in expression of all three AQPs using Anti-Aquaporin 1 Antibody, Anti-Aquaporin 2 Antibody and Anti-Aquaporin 3 Antibody9. Expression of AQP1-3 was decreased in kidneys with left obstructed ureter compared to its contralateral kidney that was left untouched. Alomone Labs antibodies directed against AQP1- 3 demonstrated diminished expression of these channels in the ureteral-obstructed kidney by immunohistochemical staining as well as by western blot analysis27.
Induced denervation in rats by painting the renal vessels with 10% phenol through a midline abdominal incision, led to a decrease in AQP2 expression, demonstrated by western blot analysis and by immunohistochemical staining using Alomone Labs respective antibody12 (Figure 2).
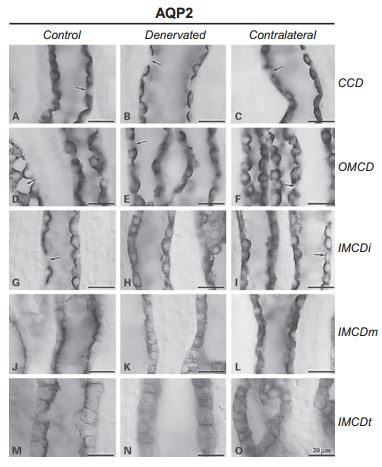
Immunohistochemical staining of AQP2 in control, denervated and contralateral kidneys using Anti-Aquaporin 2 Antibody (#AQP-002) demonstrated marked decrease in AQP2 expression in the inner medullary collecting ducts, and a slight decrease in the expression of AQP2 in the cortical and outer medullary collecting ducts. In addition, AQP2 immunoreactivity was diffusely dispersed throughout the cytoplasm with decreased apical labeling in the denervated kidney, being contrasted by prominent apical labeling in the control. CCD, cortical collecting duct; OMCD, outer medullary collecting duct; IMCDi, IMCDm, IMCDt, initial, middle, terminal parts of inner medullary collecting ducts respectively. Scale bars, 20 μm.
Adapted from reference 12 with permission of S. Karger AG.
Following renal transplantation, patients often develop tubular disorders, particularly due to (subclinical) acute rejection. Following kidney transplantation in rats, it was demonstrated that the following exhibited a decrease in expression and function: Na+/H+ Exchanger Type 3 (NHE-3) in the proximal tubule, Epithelial Na+ Channel (ENaC), and AQP2 (detected with Anti-Aquaporin 2 Antibody) in the cortical collecting ducts. These data strongly suggest that shortly after transplantation, Na+ and water imbalances occur18.
Aquaporin 6
Aquaporin 6 is predominantly expressed in intracellular vesicles in α-intercalated cells in the collecting duct of the kidney (intercalated cells, make up a third of the cells in the cortical collecting duct and mediate acid-base transport). AQP6 was found to allow permeation of anions following activation with acidic pH or Hg2+ ions.
AQP6 was shown to bind calmodulin (a Ca2+-binding protein). AQP6-expressing CHO-K1 cell lysates were mixed with calmodulin beads and AQP6 was pulled down in the presence of Ca2+ or EGTA. Immunoblot using Anti-Aquaporin 6 Antibody (#AQP-006) confirmed the presence of AQP6 in the sample, thereby confirming the binding of calmodulin to the water channel17. The binding of AQP6 to calmodulin may be important in deciphering the physiological role of AQP6 in the kidney.
Aquaporins in the Brain
Several AQPs were found to be expressed in the brain: AQP1, AQP4, AQP5 and AQP9. AQP1 was detected in epithelial cells in the choroid plexus whereas AQP4, AQP5 and AQP9 were localized in astrocytes and ependymal cells (ependymal cells are involved in the production of cerebrospinal fluid, and are the thin epithelial membrane cells lining the ventricular system of the brain and the spinal cord and are one of the four types of neuroglia in the central nervous system)3. AQP4 and AQP9 appear to be implicated in brain homeostasis and in central plasma osmolarity regulation.
Aquaporin 1
Aquaporin 1 was proposed to be the major water transporting protein in the choroid plexus. It appears to be expressed in capillary endothelial cells in the systemic circulation but not in capillaries of the rat cerebrovascular system. AQP1 immunolabeling using Anti-Aquaporin 1 Antibody, was observed in various tumor cell lines of primary glioblastoma multiforme (GBM)15 (Figure 3).

Immunohistochemical staining of primary glioblastoma multiforme (GBM) tumors (A) and normal adult brain temporal cortex (B) specimens using Anti-Aquaporin 1 Antibody (#AQP-001). AQP1 immunostaining in GBM revealed a combination of cytoplasmic and cell membrane staining, whereas normal brain was negative.
Adapted from reference 15 with permission of the American Physiological Society.
Aquaporin 4
Aquaporin 4 is the predominant AQP expressed in the brain, in the perivascular margin of astroglial cells, in the blood-brain barrier (BBB) and in ependyma and pial surfaces in contact with cerebrospinal fluid2,23. The distribution of AQP4 suggests that it provides an exit for excess brain water in sever pathophysiologic conditions. However, in brain edema, water enters the brain through the intact blood-brain barrier, following osmotic force. Therefore, in AQP4-/- mice, after experiencing water intoxication and focal cerebral schemia have reduced edema formation and survive better than wild-type mice in a model of brain edema caused by acute water intoxication14.
Neuromyelitis optica (NMO), is an autoimmune inflammatory disorder in which a person’s own immune system attacks the optic nerves and spinal cord. The targeted protein, in some patients with NMO has been identified as AQP4. Immunohistochemical staining of primate cerebellum from healthy and NMO patients, using Anti-Aquaporin 4 (AQP4) (249-323) Antibody (#AQP-004), demonstrated loss of AQP4 immunoreactivity in NMO cerebellum compared to healthy subject and also demonstrated that NMO-IgG immunoreactivity completely overlaps with AQP4 immunoreactivity25.
Aquaporins in the Lungs
Four AQPs have been identified in the respiratory tract: AQP1, AQP3, AQP4 and AQP5.
Aquaporin 1
Aquaporin 1 is expressed in the apical and basolateral membrane of the microvascular endothelium, as well as in the visceral pleura.
Aquaporin 5
Aquaporin 5 is selectively expressed in the apical plasma membrane of various secretory glands such as the airway submucosal glands, and alveolar type I epithelial cells in the lungs16,23.
It was demonstrated that nitric oxide decreases cell surface expression of AQP516. Nitric oxide (NO) is a ubiquitous molecule produced by numerous cell types and is involved in a wide range of disease processes such as lung inflammation and edema6,16. MLE-12 cells were treated with NOC-18 (a nitric oxide donor) for 2 hours and stained with Anti-Aquaporin 5 Antibody (#AQP-005)16, (Figure 4). While most of the AQP5 signal was associated with the plasma membrane (Figure 4A), staining on cell membrane was markedly decreased, and increased intracellular staining was observed compared to control cells, following treatment with NOC-1816 (Figure 4B). This signal was abolished with treatment of methyl-β-cyclodextrin, an endocytosis inhibitor (Figure 4C). The decrease in AQP5 expression along with the increase in NO metabolites, eNOS, and iNOS was also described in acute lung injury (ALI) induced by bleomycin inhalation6.
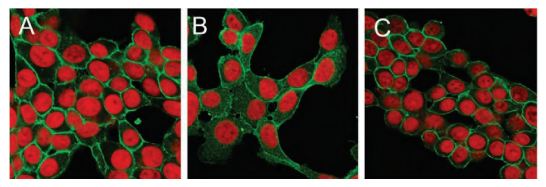
MLE-12 cells were incubated with NOC-18, and stained with Anti-Aquaporin 5 Antibody (#AQP-005), (green). In control cells, most of AQP5 signals are associated with the plasma membrane (A). After 2 hours of treatment with NOC-18, AQP5 staining on the membrane is markedly decreased, and intracellular staining is increased (B). This translocation of AQP5 staining was abolished by the use of methyl-β- cyclodextrin (C).
Adapted from reference 16 with permission of Elsevier.
Aquaporins in other Tissues
Taste Buds
Immunohistochemical staining of AQP1 and AQP2 using Anti-Aquaporin 1 Antibody and Aquaporin 2 Antibody revealed expression of both channels in rat taste buds. AQP1 and AQP2 were immunolabeled predominantly in the basolateral membrane. Double labeling demonstrated overlapping between AQP1 and AQP2 in many but not all taste cells26 (Figure 5).
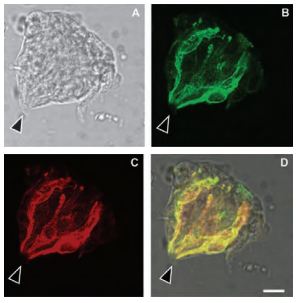
Immunohistochemical staining of rat taste buds double labeled with Anti-Aquaporin 1 Antibody (#AQP-001) and Anti-Aquaporin 2 Antibody (#AQP-002). DIC image of a vallate taste bud (A) and the same taste bud labeled with Anti-Aquaporin 1 Antibody (green, B) and Anti-Aquaporin 2 Antibody (red, C). D) Merged images, highlighting the lack of apical labeling in these taste buds (arrowhead). Scale bars, 10 μm.
Adapted from reference 26 with permission of Oxford University Press.
Cornea and Retina
In the cornea, AQP1 is found in the endothelial cells and has been shown to function in osmotic water transport in mice.
The effect of ozonated solution on the cornea morphology was studied, where AQP1 and ZO-1 (tight junction-associated protein) expression was tested along with the protective effect of ascorbic acid (AA). Immunohistochemical staining using Anti-Aquaporin 1 Antibody, showed that the expression of AQP1 in the AA group was similar to that in the control21.
In the retina, AQP4 is expressed in Müller cells (associated with bipolar cells). AQP4 expression in Müller cells from AQP4+/+ and AQP4-/- mice was compared using Anti-Aquaporin 4 (AQP4) (249-323) Antibody19. Depletion of Dp71, a cytoskeleton protein associated to the membrane, leads to physiological alterations in Müller cells which are similar to those observed in injured or diseased retinas. This involves a mislocation of K+ and water channels (Kir4.1 and AQP4) demonstrated by immunohistochemical staining and western blot analyses using Anti-Aquaporin 4 (AQP4) (249-323) Antibody and Anti-Kir4.1 (KCNJ10) Antibody (#APC-035). AQP4 mislocation resulted in dysregulation of water transport through Müller cells20.
New Aquaporin Antibodies
Aquaporin 7
Aquaporin 7 is permeable to water, urea and glycerol. It is expressed in a number of tissues; ovary, testis, kidney, adipose tissue and islet cells. The effect of urea and glycerol on rat pancreatic β-cell membrane potential, cell volume and insulin secretion was investigated. Immunohistochemical staining and western blot analyses assessed AQP7 expression and localization in these cells5 (Figure 6). It was concluded that glycerol and urea can activate β-cells via their rapid uptake across the β-cell plasma membrane, possibly via AQP7 resulting in cell swelling, VRAC (volume-regulated anion channel) activation, electrical activity leading to depolarization, and insulin release. Glycerol appears to exert an additional effect, possibly related to its intracellular metabolism.
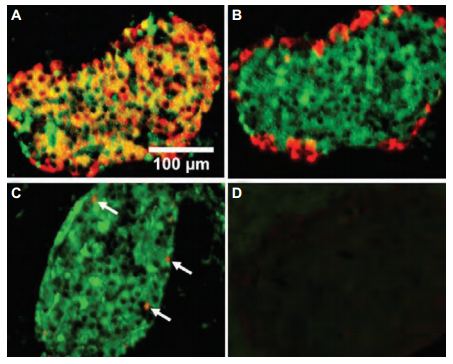
Immunohistochemical staining of rat pancreatic β-cells, double labeled with Anti-Aquaporin 7 Antibody (#AQP-007), insulin and glucagon. A) AQP7 labeling (green) overlaps with that of insulin (red), but not with that of glucagon (red, B). C) AQP7 labeling (green) overlaps with that of somatostatin (in red, indicated by arrows). D) Negative control performed in the absence of primary antibodies. Scale bar, 100 μm.
Adapted from reference 5 with permission of S. Karger AG.
Aquaporin 8
Aquaporin 8 is expressed in the liver, pancreas, intestine, salivary gland, testes and heart and is primarily water selective. Immunohistochemical staining of rat pancreas and lung demonstrated the expression of AQP8 in both tissues, using Anti-Aquaporin 8 Antibody (#AQP-008), (Figure 7).
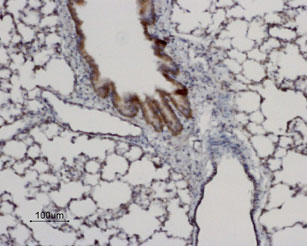
Immunohistochemical staining of AQP8 in rat lung using Anti-Aquaporin 8 Antibody (#AQP-008). AQP8 labeling (brown) is specific for the bronchiolar epithelium. Hematoxilin is used as the counterstain.
Experimental procedure and figure processed at Alomone Labs Ltd.
Aquaporin 9
Aquaporin 9 is expressed in the liver, white blood cells, testis and brain. It is permeable to water and small solutes. Immunohistochemical staining of rat liver and testis demonstrated the expression of AQP9 in both tissues, using Anti-Aquaporin 9 Antibody (#AQP-009), (Figure 8).
Alomone Labs is following with great interest after the developing research of Aquaporins and keeps updating and offering new antibodies, intracellular as well as extracellular, to meet research needs.

Immunohistochemical staining of AQP9 in rat testes using Anti-Aquaporin 9 Antibody (#AQP-009). AQP9 labeling (brown) appears in the columnar epithelium of the epididymus (arrows). Hematoxilin is used as the counterstain.
Experimental procedure and figure processed at Alomone Labs Ltd.
References
- Agre, P. et al. (2002) J. Physiol. 542.1, 3.
- Agre, P. (2000) J. Am. Soc. Nephrol. 11, 764.
- Badaut, J. et al. (2002) J. Cereb. Blood Flow Metab. 22, 367.
- Bae, E.H. et al. (2009) Nephrol. Dial. Transplant. 24, 2692.
- Best, L. et al. (2009) Cell. Physiol. Biochem. 23, 255.
- Jung, A.S. et al. (2004) Intensive Care Med. 30, 489.
- Kang, D.G. et al. (2004) Biol. Pharm. Bull. 27, 366.
- Kim, S.W. et al. (2001) J. Am. Soc. Nephrol. 12, 875.
- Kim, S.W. et al. (2001) J. Am. Soc. Nephrol. 12, 2019.
- Lee, J. et al. (2000) Clin. Exp. Hyper. 22, 531.
- Lee, J. et al. (2006) Kidney Blood Press. Res. 29, 18.
- Lee, J. et al. (2006) Nephron Physiol. 103, 170.
- Ma, T. et al. (1998) J. Biol. Chem. 273, 4296.
- Manley, J.T. et al. (2000) Nat. Med. 6, 159.
- Markert, J.M. et al. (2001) Physiol. Genomics 5, 21.
- Nagai, K. et al. (2007) Biochem. Biophys. Res. Commun. 354, 579.
- Rabaud, N.E. et al. (2009) Biochem. Biophys. Res. Commun. 383, 54.
- Reuter, S. et al. (2008) Eur. J. Physiol. 456, 1075.
- Ruiz-Ederra, J. et al. (2007) J. Biol. Chem. 282, 21866.
- Sene, A. et al. (2009) PLoS One 4, e7329.
- Suzuki, H. et al. (2009) Jpn. J. Ophthalmol. 53, 151.
- Valenti, G. et al. (2005) Endocrinology 146, 5063.
- Verkman, A.S. (2005) J. Cell Sci. 118, 3225.
- Verkman, A.S. (2009) J.Exp. Biol. 212, 1707.
- Vincent, T. et al. (2008) J. Immunol. 181, 5730.
- Watson, K.J. et al. (2007) Chem. Senses 32, 411.
- Yeum, C.H. et al. (2003) Scand. J. Urol. Nephrol. 37, 99.