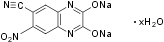
Overview
- Honore, T. et al. (1988) Science 241, 701.
- Traynelis, S.F. et al. (2010) Pharmacol. Rev. 62, 405.
 Alomone Labs CNQX disodium salt inhibits GluA1 channels expressed in Xenopus oocytes.Time course of current reversible inhibition by 20, 2 and 10 μM CNQX disodium salt (#C-141) (at -80 mV) in the presence of 100 μM glutamate. Amplitude change as a result of the application of different concentrations of CNQX disodium salt (perfusion duration indicated by the horizontal bar, concentration as indicated).
Alomone Labs CNQX disodium salt inhibits GluA1 channels expressed in Xenopus oocytes.Time course of current reversible inhibition by 20, 2 and 10 μM CNQX disodium salt (#C-141) (at -80 mV) in the presence of 100 μM glutamate. Amplitude change as a result of the application of different concentrations of CNQX disodium salt (perfusion duration indicated by the horizontal bar, concentration as indicated).
- Honore, T. et al. (1988) Science 241, 701.
- Traynelis, S.F. et al. (2010) Pharmacol. Rev. 62, 405.
- Long, S.K. et al. (1990) Br. J. Pharmacol. 100, 850.
- Watkins, J.C. et al. (1990) Trends Pharmacol. Sci. 11, 25.
The first widely used competitive AMPA receptor antagonists were quinoxalinediones (CNQX, DNQX, NBQX), which were highly selective over NMDA receptors but antagonized kainate receptors. CNQX is a potent, competitive AMPA/kainate receptor antagonist. It also acts as an antagonist at the NMDA receptor glycine site.
This non-NMDA receptor antagonist inhibits [3H]AMPA binding to quisqualate receptors at submicromolar concentrations. CNQX also selectively blocks the excitatory action of quisqualate and kainate on spinal neurons with little or no effect on that of NMDA.
The concentrations that inhibit 50% of [3H]AMPA binding (IC50) for CNQX in dorsal horn neurons is 300 nM1.
The association of AMPA receptors with TARP auxiliary subunits converts CNQX from an antagonist to a weak partial agonist. CNQX induces partial domain closure, consistent with the activity of a partial agonist. CNQX blocks both fast AMPA-mediated and slow kainate receptor-mediated mEPSCs2.
EC50 values for depression of the monosynaptic ventral root reflex is 1.0 ± 0.3 µM3.
Direct binding studies using CNQX as a radioligand show that CNQX binds with high affinity (40 nM) to both high (14 nM) and low (235 nM) affinity AMPA binding sites in rat brain4.
CNQX disodium salt (#C-141) is a highly pure, synthetic, and biologically active compound.
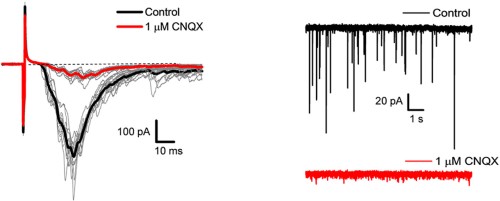
Alomone Labs CNQX disodium salt abolishes glutamatergic synaptic currents in rat dorsal horn.Whole-cell patch-clamp recordings on spinal cord neurons. Monosynaptic evoked EPSCs (eEPSCs) of C- and Aδ-fibers of the dorsal root were sensitive to 1 µM CNQX disodium salt (#C-141), (left) indicating that glutamatergic synaptic transmission is likely associated with nociceptive signaling. In the absence of stimulation (right), spontaneous glutamatergic synaptic currents were also observed and were sensitive to CNQX disodium salt.Adapted from Muqeem, T. et al. (2018) J. Neurosci. 38, 3729. with permission of the Society for Neuroscience.
Applications
Citations
- Muqeem, T. et al. (2018) J. Neurosci. 38, 3729.
- Tang, Y. et al. (2018) Eur. J. Neurosci. 47, 866.