Overview
Cat #:
D-350
Alternative Name Venom basic protease inhibitor 1 homolog, Toxin C13S2C3, α-DTX
Lyophilized Powder yes
Origin Natural peptide isolated from Dendroaspis angusticeps (Eastern green mamba).
MW: 7048 Da
Purity: >98% (HPLC)
Effective concentration 10-500 nM.
Sequence QPRRKLCILHRNPGRCYDKIPAFYYNQKKKQCERFDWSGCGGNSNRFKTIEECRRTCIG.
Modifications Disulfide bonds between Cys7-Cys57, Cys16-Cys40 and Cys32-Cys53. Gln1 – Pyrrolidone carboxylic acid.
Molecular formula C305H481N99O84S6.
CAS No.: 74504-53-3
Activity α-Dendrotoxin inhibits 4-AP sensitive, inactivating voltage-gated K+ channels (KV1.1, KV1.2 and KV1.6).
Shipping and storage Shipped at room temperature. Product as supplied can be stored intact at room temperature for several weeks. For longer periods, it should be stored at -20°C.
Solubility Any aqueous buffer. Centrifuge all product preparations before use (10000 x g 5 min).
Storage of solutions Up to four weeks at 4°C or three months at -20°C.
Our bioassay
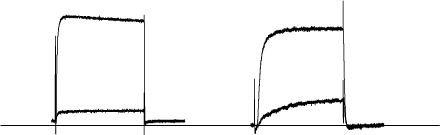 Alomone Labs α-Dendrotoxin inhibits KV1.1 and KV1.2 channel currents heterologously expressed in Xenopus oocytes.KV1.1 (left, in 2 mM K+) and KV1.2 (right, in 5 mM K+) channel currents elicited by 200 ms depolarization from a holding potential of -100 mV to +20 mV, before and during application of 100 nM α-Dendrotoxin (#D-350). 73% (n = 4) of the KV1.1 and 80% (n = 4) of the KV1.2 channels were inhibited by α-Dendrotoxin, respectively.
Alomone Labs α-Dendrotoxin inhibits KV1.1 and KV1.2 channel currents heterologously expressed in Xenopus oocytes.KV1.1 (left, in 2 mM K+) and KV1.2 (right, in 5 mM K+) channel currents elicited by 200 ms depolarization from a holding potential of -100 mV to +20 mV, before and during application of 100 nM α-Dendrotoxin (#D-350). 73% (n = 4) of the KV1.1 and 80% (n = 4) of the KV1.2 channels were inhibited by α-Dendrotoxin, respectively.
References - Scientific background
- Harvey, A.L. et al. (1980) Naunyn Schmeidebergs Arch. Pharmacol. 312, 1.
- Benishin, C.G. et al. (1988) Mol. Pharmacol. 34, 152.
- Harvey, A.L. and Anderson, A.J. (1985) Pharmacol. Ther. 31, 33.
- Harvey, A.L. (2001) Toxicon 39, 15.
- Baez, A. et al. (2015) Neurosci. Lett. 606, 42.
Scientific background
α-Dendrotoxin is isolated from Dendroaspis angusticeps snake venom by modification of the procedures of Harvey1 and Benishin2 and purified to homogeneity. α-Dendrotoxin blocks KV1.1 and KV1.2 channels (IC50= 0.4 to 4 and 1.1 to 12 nM in oocytes respectively, with higher values for mammalian cells).4 In addition, the toxin was shown to block KV1.6 (IC50= 9-25 nM).4
α-Dendrotoxin was recently found to block ASIC currents in rat dorsal root ganglia with IC50 in the nM range5.
Target KV1.1, KV1.2, KV1.6, ASIC channels
Peptide Content: 100%
Lyophilized Powder
α-Dendrotoxin (#D-350) is a highly pure, natural, and biologically active peptide toxin.
Image & Title 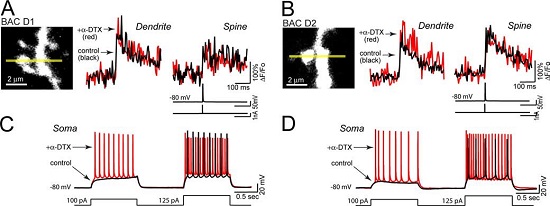
Alomone Labs α-Dendrotoxin inhibits KV1.2 channel currents in somatic MSN cells.Line scans taken 45–60 µm from the soma show that blockade of KV1.2 channels with α-Dendrotoxin (#D-350) (0.5 µM) does not affect the amplitude of the bAP-evoked Ca2+ transient in dendrites (left traces in A and B) or adjacent spines (right traces in A and B) of either D1 or D2 MSNs (control: black, α-Dendrotoxin: red n = 5 each). Somatic voltage recordings from the MSNs shown in A) and B) demonstrate that spiking is enhanced by α-Dendrotoxin in both D1 and D2 MSNs (control: black, α-Dendrotoxin: red). Recordings were generated by injecting sequential depolarizing current steps at amplitudes just before and after rheobase potentials (100 and 125 pA, lower black line), (C and D).Adapted from Day, M. et al. (2008) J. Neurosci. 28, 11603. with permission of the Society for Neuroscience.

Alomone Labs α-Dendrotoxin inhibits KV1.2 channel currents in somatic MSN cells.Line scans taken 45–60 µm from the soma show that blockade of KV1.2 channels with α-Dendrotoxin (#D-350) (0.5 µM) does not affect the amplitude of the bAP-evoked Ca2+ transient in dendrites (left traces in A and B) or adjacent spines (right traces in A and B) of either D1 or D2 MSNs (control: black, α-Dendrotoxin: red n = 5 each). Somatic voltage recordings from the MSNs shown in A) and B) demonstrate that spiking is enhanced by α-Dendrotoxin in both D1 and D2 MSNs (control: black, α-Dendrotoxin: red). Recordings were generated by injecting sequential depolarizing current steps at amplitudes just before and after rheobase potentials (100 and 125 pA, lower black line), (C and D).Adapted from Day, M. et al. (2008) J. Neurosci. 28, 11603. with permission of the Society for Neuroscience.
For research purposes only, not for human use
Last Update: 07/05/2024
Applications
Citations
Citations
Electrophysiology
- Mouse brain slices (100 nM).
Lahiri, A. and Bevan, M.D. (2020) Neuron 106, 1.
Product citations
- Muqeem, T. et al. (2018) J. Neurosci. 38, 3729.
- Hou, W.H. et al. (2016) J. Neurosci. 36, 4549.
- Meneses, D. et al. (2016) Neural Plast. 2016, 8782518.
- Pathak, D. et al. (2016) J. Neurophysiol. 115, 2317.
- Casale, A.E. et al. (2015) J. Neurosci. 35, 15555.
- Giglio, A.M. and Storm, J.F. (2014) Eur. J. Neurosci. 39, 12.
- Yang, J. et al. (2013) J. Physiol. 591, 3233.
- Bocksteins, E. et al. (2012) Am J. Physiol. 303, C406.
- Saito, Y. et al. (2012) Neurosci. Res. 73, 32.
- Alle, H. et al. (2011) J. Neurosci. 31, 8001.
- Casale, A.E. and McCormick, D.A. (2011) J. Neurosci. 31, 18289.
- Fulton S. et al. (2011) J. Biol. Chem. 286, 9360.
- Guan, D. et al. (2011) J. Neurophysiol. 106, 1722.
- Martel, P. et al. (2011) PLoS ONE 6, e20402.
- Min, M.Y. et al. (2010) Neuroscience 168, 633.
- Norris, A.J. and Nerbonne, J.M. (2010) J. Neurosci. 30, 5092.
- Guzman, J.N. et al. (2009) J. Neurosci. 29, 11011.
- Iremonger, K.J. and Bains, J.S. (2009) J. Neurosci. 29, 7349.
- Madrid, R. et al. (2009) J. Neurosci. 29, 3120.
- Menteyne, A. et al. (2009) PLoS ONE 4, e6770.
- Petreanu, L. et al. (2009) Nature 457, 1142.
- Wu, Z.Z. et al. (2009) J. Biol. Chem. 284, 36453.
- Cho, K.H. et al. (2008) J. Neurophysiol. 99, 2833.
- Day, M. et al. (2008) J. Neurosci. 28, 11603.
- Povysheva, N.V. et al. (2008) J. Neurophysiol. 100, 2348.

