Overview
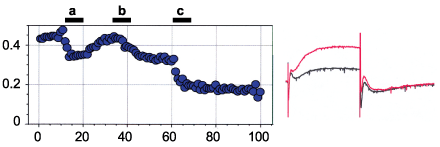 Alomone Labs Paxilline inhibits KCa1.1 (BK) channels heterologously expressed in Xenopus oocytes.Left: The effect of 100 nM Paxilline (#P-450) (a), Penitrem A (P-650) (b), and Verruculogen (#V-500) (c) on BK currents. Time course of current amplitude changes upon sequential application of the three toxins. Right: Example of current responses to 100 ms depolarization before (red) and during (black) perfusion of 500 nM Paxilline.
Alomone Labs Paxilline inhibits KCa1.1 (BK) channels heterologously expressed in Xenopus oocytes.Left: The effect of 100 nM Paxilline (#P-450) (a), Penitrem A (P-650) (b), and Verruculogen (#V-500) (c) on BK currents. Time course of current amplitude changes upon sequential application of the three toxins. Right: Example of current responses to 100 ms depolarization before (red) and during (black) perfusion of 500 nM Paxilline.
- Selala, M.I. et al. (1991) J. Nat. Prod. 54, 207.
- Knaus, H.G. et al. (1994) Biochemistry 33, 5819.
- Modulator Issue No. 17.pdf
- Hu, H. et al. (2001) J. Neurosci. 21, 9585.
- Faber, E.S. and Sah, P. (2002) J. Neurosci. 22, 1618.
- Bilmen, J.G. et al. (2002) Arch. Biochem. Biophys. 406, 55.
Paxilline is a selective blocker of KCa1.1 (high-conductance Ca2+-activated K+, BK) channels. It belongs to a set of tremorgenic indole alkaloids which are lipid soluble and also include Penitrem A and Verruculogen.2
Paxilline enhanced the electrically induced twitch contractions in guinea pig ileum, without influencing the contractions provoked by exogenous acetylcholine.1 Paxilline was shown to inhibit the peptidyle toxin resistant combination a+b4 channels (for more background see 3) expressed in CHO cells.4 It was shown to be as potent as Iberiotoxin in action potential broadening in hippocampus and amygdala.4,5
Paxilline also inhibits intracellular Ca2+-ATPases (SERCA) with IC50s between 5 and 10 µM6.
Paxilline (#P-450) is a highly pure, natural, and biologically active compound.
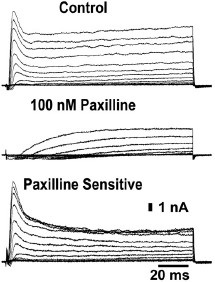
Alomone Labs Paxilline inhibits KCa1.1 channel currents in isolated canine intracardiac neurons.Representative current traces show a total blockage of the fast-activated current component and a reduction of the slow outward component caused by 100 nM Paxilline (#P-450).Adapted from Perez, G.J. et al. (2013) Am. J. Physiol. 304, C280. with permission of The American Physiological Society.
Applications
Citations
- Iordache, F. et al. (2016) J. Physiol. Sci. 66, 463.
- Zhang, Y.Y. et al. (2014) J. Cell. Phys. 229, 202.
- Chen, M. et al. (2013) Mol. Neurobiol. 48, 794.
- Perez, G.J. et al. (2013) Am. J. Physiol. 304, C280.
- Keating, N. and Quinlan, L.R. (2012) Am. J. Physiol. 302, C100.
- Ishii, H. et al. (2010) Eur. J. Neurosci. 32, 736.
- Henney, N.C. et al. (2009) Am. J. Physiol. 297, C1397.
- Griguoli, M. et al. (2009) Eur. J. Neurosci. 30, 1011.
- Benhassine, N. and Berger, T. (2009) Pflugers Arch. 457, 1133.
- Liu, Y.C. et al. (2009) Naunyn-Schmiedeberg's Arch. Pharmacol. 379, 127.
- Wu, S.N. et al. (2008) Mol. Pharmacol. 74, 1696.