Overview
Cat #:
STC-325
Alternative Name K+ channel toxin α-KTx 1.1, ChTX, ChTX-Lq1, ChTx-a
Lyophilized Powder yes
Origin Synthetic peptide
MW: 4296 Da
Purity: >98% (HPLC)
Effective concentration 10-100 nM.
Sequence ZFTNVSCTTSKECWSVCQRLHNTSRGKCMNKKCRCYS.
Modifications Disulfide bonds between Cys7-Cys28, Cys13-Cys33, and Cys17-Cys35, Z= Pyrrolidone carboxylic acid.
Molecular formula C176H277N57O55S7.
CAS No.: 95751-30-7
Activity Charybdotoxin is a potent selective inhibitor of high conductance (maxi-K), different medium and small conductance Ca2+-activated K+ channels, as well as a voltage-dependent K+ channel (KV1.3)1.
References-Activity
- Gimenez-Gallego, G. et al. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 3329.
Accession number P13487.
Shipping and storage Shipped at room temperature. Product as supplied can be stored intact at room temperature for several weeks. For longer periods, it should be stored at -20°C.
Solubility Any aqueous buffer. Centrifuge all product preparations before use (10000 x g 5 min).
Storage of solutions Up to two weeks at 4°C or three months at -20°C.
Our bioassay
 Alomone Labs Charybdotoxin inhibits KCa1.1 channels heterologously expressed in Xenopus oocytes.A. Example of time course showing reversible effect of 20 nM and 50 nM Charybdotoxin (#STC-325) during 100 sec application on the current amplitude. Membrane holding potential was -100 mV stepped to 0 mV during 200 ms following another step to 80 mV during 600 msec. B. Superimposed example traces of KCa1.1 channel currents in response to ramp depolarization before (Control) and during the application of 20 nM or 50 nM of Charybdotoxin for 100 sec.
Alomone Labs Charybdotoxin inhibits KCa1.1 channels heterologously expressed in Xenopus oocytes.A. Example of time course showing reversible effect of 20 nM and 50 nM Charybdotoxin (#STC-325) during 100 sec application on the current amplitude. Membrane holding potential was -100 mV stepped to 0 mV during 200 ms following another step to 80 mV during 600 msec. B. Superimposed example traces of KCa1.1 channel currents in response to ramp depolarization before (Control) and during the application of 20 nM or 50 nM of Charybdotoxin for 100 sec.
References - Scientific background
- Gimenez-Gallego, G. et al. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 3329.
- Miller, C. et al. (1985) Nature 313, 316.
- Grissmer, S. et al. (1994) Mol. Pharmacol. 45, 1227.
- Meera, P. et al. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5562.
- Sugg, E.E. et al. (1990) J. Biol. Chem. 265, 18745.
Scientific background Charybdotoxin was originally isolated from the venom of the Israeli scorpion Leiurus quinquestriatus hebraeus1. Charybdotoxin blocks KCa1.1 (large conductance Ca2+-activated K+, Slo) channels in nM concentrations2 as well as KV1.2 (Kd, 14 nM) and KV1.3 (Kd, 2.6 nM) channels3. However, experiments with cloned KCa1.1 channels demonstrate the strong effect of the sloβ subunits on the potency of block by Charybdotoxin (see for example 4).
Target KCa1.1, KV1.2, KV1.3 K+ channels
Peptide Content: 100%
Lyophilized Powder
Charybdotoxin (#STC-325) is a highly pure, synthetic, and biologically active peptide toxin.
Image & Title 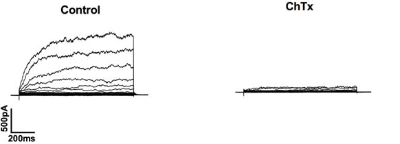
Alomone Labs Charybdotoxin blocks BKCa channels in human chorionic plate arterial smooth muscle cells.Inhibition of BKCa channels with Charybdotoxin (#STC-325) which abolished outward currents at +80 mV. Cells were voltage clamped at -60 mV and step depolarised from -70 mV to +80 mV for 500 ms in 10 mV increments and repolarised to -40 mV.Adapted from Brereton, M.F. et al. (2013) PLoS ONE 8, e57451. with permission of PLoS.

Alomone Labs Charybdotoxin blocks BKCa channels in human chorionic plate arterial smooth muscle cells.Inhibition of BKCa channels with Charybdotoxin (#STC-325) which abolished outward currents at +80 mV. Cells were voltage clamped at -60 mV and step depolarised from -70 mV to +80 mV for 500 ms in 10 mV increments and repolarised to -40 mV.Adapted from Brereton, M.F. et al. (2013) PLoS ONE 8, e57451. with permission of PLoS.
For research purposes only, not for human use
Last Update: 07/05/2024
Applications
Citations
Citations
Product citations
- Fellerhoff-Losch, B. et al. (2015) J. Neural Transm. 123, 137.
- Lee, J. et al. (2014) Develop. Neurobiol. 74, 1.
- Lopez-Gonzalez, I. et al. (2014) Mol. Hum. Reprod. 20, 619.
- Brereton, M.F. et al. (2013) PLoS ONE 8, e57451.
- Cao, Z. et al. (2013) Nat. Commun. 4, 1.
- Chen, M. et al. (2013) Mol. Neurobiol. 48, 794.
- Gonzalez Corrochano, R. et al. (2013) Br. J. Pharmacol. 169, 449.
- Cosyns, S.M. and Lefebvre, R.A. (2012) Eur. J. Pharmacol. 686, 104.
- Sun, P. et al. (2011) J. Neurosci. 31, 16464.
- Tanner, G.R. et al. (2011) J. Neurosci. 31, 8689.
- Malinina, E. et al. (2010) J. Neurophysiol. 104, 200.
- Sciaccaluga, M. et al. (2010) Am. J. Physiol. 299, C175.
- Dhaese, I. and Lefebvre, R.A. (2009) Eur. J. Pharmacol. 606, 180.
- Begg, M. et al. (2001) J. Physiol. 531, 95.

