Overview
Cat #:
T-650
Lyophilized Powder yes
Origin Thapsia garganica.
Source Natural
MW: 650.7
Purity: >99% (HPLC)
Effective concentration 50 nM - 1 μM.
Chemical name (3S,3aR,4S,6S,6AR,7S,8S,9bS)-6-(Acetyloxy)-2,3,3a,4,5,6 ,6a,7,8,9b-decahydro-3,3a-dihydroxy-3,6,9-trimethyl-8-[ [(2Z)-2-methyl-1-oxo-2-butenyl]oxy]-2-oxo-4-(1-oxobutox y)azuleno[4,5-b]furan-7-yl octanoate.
Molecular formula C34H50O12.
CAS No.: 67526-95-8
Activity Thapsigargin is a highly potent inhibitor of the sarco-endoplasmic reticulum Ca2+-ATPases (SERCAs), inhibiting Ca2+ pumping to intracellular stores at nanomolar concentrations, with IC50 ranging from 0.2 nM to 50 nM in various mammalian cell types.1 Thapsigargin-induced depletion of Ca2+ stores leads to apoptosis in various cell lines.2-4
References-Activity
- Rodriguez-Lopez, A.M. et al. (1999) Cell Physiol. Biochem. 9, 285.
- Wei, H. et al. (1998) J. Neurochem. 70, 2305.
- Waring, P. and Beaver, J. (1996) Exp. Cell. Res. 227, 264.
- Jackson, T.R. et al. (1988) Biochem. J. 253, 81.
Shipping and storage Shipped at room temperature. Product as supplied can be stored intact at room temperature for several weeks. For longer periods, it should be stored at -20°C.
Solubility DMSO, ethanol or acetone. Centrifuge all product preparations before use (10000 x g 5 min).
Storage of solutions Up to one week at 4°C or three months at -20°C.
Our bioassay
 Alomone Labs Thapsigargin induces cytosolic Ca2+ increase in Jurkat cells.Cells were loaded with Fluo-3-AM dye in the presence of 5 mM EGTA. 20 nM Thapsigargin (#T-650) application induces a significant increase in intracellular Ca2+. Changes in intracellular Ca2+ were detected as changes in Fluo-3 fluorescent emission following the application (arrow) of control or Thapsigargin.
Alomone Labs Thapsigargin induces cytosolic Ca2+ increase in Jurkat cells.Cells were loaded with Fluo-3-AM dye in the presence of 5 mM EGTA. 20 nM Thapsigargin (#T-650) application induces a significant increase in intracellular Ca2+. Changes in intracellular Ca2+ were detected as changes in Fluo-3 fluorescent emission following the application (arrow) of control or Thapsigargin.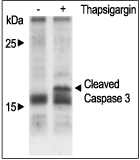 Alomone Labs Thapsigargin induces apoptosis in Jurkat cells.Cells were grown to 70% confluency. 1 μM Thapsigargin (#T-650) or vehicle was added for six hours. Cell extracts were then probed for cleaved Caspase 3 with specific antibodies.
Alomone Labs Thapsigargin induces apoptosis in Jurkat cells.Cells were grown to 70% confluency. 1 μM Thapsigargin (#T-650) or vehicle was added for six hours. Cell extracts were then probed for cleaved Caspase 3 with specific antibodies. Alomone Labs Thapsigargin increases intracellular Ca2+ by modulating the autophosphorylation of CaMK II in 3T3-L1 cells.Cells were starved for 2h and then stimulated with 1 or 5 µM Thapsigargin (#T-650) for the indicated times. The cell extracts were blotted and probed with an antibody for phospho-(Thr268)-CAMKII (Calmodulin dependent kinase II).
Alomone Labs Thapsigargin increases intracellular Ca2+ by modulating the autophosphorylation of CaMK II in 3T3-L1 cells.Cells were starved for 2h and then stimulated with 1 or 5 µM Thapsigargin (#T-650) for the indicated times. The cell extracts were blotted and probed with an antibody for phospho-(Thr268)-CAMKII (Calmodulin dependent kinase II). Alomone Labs Thapsigargin induces cytosolic Ca2+ increase in HEK 293 cells.Ca2+ traces from Fluo-3 AM loaded HEK 293 cells treated with 5 µM Thapsigargin (#T-650) in the presence of 5 mM EGTA.
Alomone Labs Thapsigargin induces cytosolic Ca2+ increase in HEK 293 cells.Ca2+ traces from Fluo-3 AM loaded HEK 293 cells treated with 5 µM Thapsigargin (#T-650) in the presence of 5 mM EGTA.
References - Scientific background
- Treiman, M. et al. (1998) Trends Pharmacol. Sci. 19, 131.
- Rodriguez-Lopez, A.M. et al. (1999) Cell Physiol. Biochem. 9, 285.
- Waring, P. and Beaver, J. (1996) Exp. Cell. Res. 227, 264.
- Wei, H. et al. (1998) J. Neurochem. 70, 2305.
- Christensen, S.B. et al. (1980) Tetrahedron Lett. 21, 3829.
- Christensen, S.B. et al. (1982) J. Org. Chem. 47, 649.
- Rasmussen, U. et al. (1978) Acta Pharm. Suec. 15, 133.
- Jackson, T.R. et al. (1988) Biochem. J. 253, 81.
- Sagara, Y. and Inesi, G. (1991) J. Biol. Chem. 266, 13503.
- Lytton, J. et al. (1991) J. Biol. Chem. 266, 17067.
- Takemura, H. et al. (1989) J. Biol. Chem. 264, 12266.
- Patkar, S.A. (1979) Agents Actions 9, 53.
- Ohuchi, K. et al. (1987) J. Cancer Res. Clin. Oncol. 113, 319.
Scientific background Thapsigargin, derived from the plant genus Thapsia,1-3 is an extremely tight-binding inhibitor of intracellular Ca2+ pumps. It was initially described as a tumor promoting agent which induces rapid Ca2+ release from intracellular stores4 by inhibition of the sarcoplasmic/endoplasmic reticulum Ca2+-dependent ATPase pump without inositol phosphate formation.5-7 The thapsigargin induced depletion of Ca2+ stores causes apoptosis in most cell lines.8-10 It has also been shown to cause histamine secretion from rat mast cells,11 and to stimulate arachidonic acid metabolism in macrophages.12 Its tumour-promoting function probably results, at least partly, from cytotoxicity, causing a wound response in the skin.13 The tumorogenic activity of thapsigargin might be due to its activation of protein kinase B (Akt) which subsequently stimulates MAP kinase signaling via Src and Raf-1.12,13
Target SERCA
Lyophilized Powder
Thapsigargin (#T-650) is a highly pure, natural and biologically active compound.
Image & Title 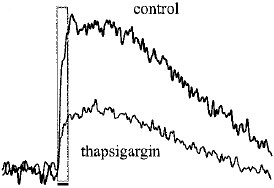 Alomone Labs Thapsigargin decreases Ca2+ signals in rat mossy fibers.Rat hippocampal slices were incubated with 10 µM Thapsigargin (#T-650) for 60 min. The Ca2+ signals from mossy fiber terminals decreased significantly after wash-in.Adapted from Liang, Y. et al. (2002) J. Neurophysiol. 87, 1132. with permission of The American Physiological Society.
Alomone Labs Thapsigargin decreases Ca2+ signals in rat mossy fibers.Rat hippocampal slices were incubated with 10 µM Thapsigargin (#T-650) for 60 min. The Ca2+ signals from mossy fiber terminals decreased significantly after wash-in.Adapted from Liang, Y. et al. (2002) J. Neurophysiol. 87, 1132. with permission of The American Physiological Society.
 Alomone Labs Thapsigargin decreases Ca2+ signals in rat mossy fibers.Rat hippocampal slices were incubated with 10 µM Thapsigargin (#T-650) for 60 min. The Ca2+ signals from mossy fiber terminals decreased significantly after wash-in.Adapted from Liang, Y. et al. (2002) J. Neurophysiol. 87, 1132. with permission of The American Physiological Society.
Alomone Labs Thapsigargin decreases Ca2+ signals in rat mossy fibers.Rat hippocampal slices were incubated with 10 µM Thapsigargin (#T-650) for 60 min. The Ca2+ signals from mossy fiber terminals decreased significantly after wash-in.Adapted from Liang, Y. et al. (2002) J. Neurophysiol. 87, 1132. with permission of The American Physiological Society.For research purposes only, not for human use
Last Update: 21/04/2024
Applications
Citations
Citations
Electrophysiology
- Mouse α-cells (single cell).
Dickerson, M.T. et al. (2019) Am. J. Physiol. 316, E646.
Product citations
- Kostic, M. et al. (2018) Cell Rep. 25, 3465.
- Maggio, N. and Vlachos, A. (2018) J. Mol. Med. 96, 1039.
- Smaardijk, K.S. et al. (2018) Biochim. Biophys. Acta Mol. Cell Res. 1865, 855.
- Whitt, J.P. et al. (2018) J. Gen. Physiol. 150, 259.
- Bittremieux, M. et al. (2017) Cell Calcium 62, 60.
- Chen, J. et al. (2017) J. Biol. Chem. 292, 6938.
- Fung-Leung, W.P. et al. (2017) PLoS ONE 12, e0170102.
- Makitani, K. et al. (2017) J. Pharmacol. Sci. 134, 37.
- Smaardijk, S. et al. (2017) Tissue Cell 49, 141.
- Vierra, N.C. et al. 92017) Sci. Signal. 10, eaan2883.
- Choi, S.Y. et al. (2016) PLoS ONE 11, e0150921.
- Landowski, L.M. et al. (2016) J. Biol. Chem. 291, 1092.
- Srivats, S. et al. (2016) J. Cell Biol. 213, 65.
- Murray, J.K. et al. (2015) J. Med. Chem. 58, 6784.
- Vutthasathien, P. and Wattanapermpool, J. (2015) J. Appl. Physiol. 119, 831.
- Mariqueo, T.A. et al. (2014) J. Neurophysiol. 111, 1940.
- Tamura, A. et al. (2014) PLoS ONE 9, e85351.
- Hooper, J.S. et al. (2013) Brain Res. 1503, 7.
- Lin, F.F. et al. (2013) J. Pharmacol. Exp. Ther. 345, 225.
- Munoz, E. et al. (2013) Cell Calcium 54, 375.
- Saleem, H. et al. (2013) PLoS ONE 8, e54877.
- Witayavanitkul, N. et al. (2013) Am. J. Physiol. 304, H465.
- Bader, P. et al. (2012) Pflugers Arch. 464, 249.6251.
- Mitchell, C.B. et al. (2012) J. Neurochem. 122, 1155.
- Shpak, G. et al. (2012) J. Neurosci. 32, 6251.
- Korkotian, E. and Segal, M. (2011) J. Physiol. 589.24, 5987.
- Fekete, A. et al. (2009) J. Neurochem. 111, 745.
- Tanaka, M. et al. (2009) J. Neurosci. Res. 87, 820.
- Hall, A.A. et al. (2009) Glia 57, 744.
- Herrera, Y. et al. (2008) J. Pharmacol. Exp. 327, 491.
- Fajardo, O. et al. (2008) J. Neurosci. 28, 7863.

