Overview
- Vriens, J. et al. (2009) Mol. Pharmacol. 75, 1262.
- Correll, C.C. et al. (2004) Neurosci. Lett. 370, 55.
- Madrid, R. et al. (2006) J. Neurosci. 26, 12512.
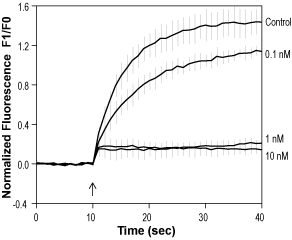 Alomone Labs BCTC blocks TRPV1 channels expressed in C6 cells.Pre-incubation with 0.1 nM, 1 nM and 10 nM BCTC (#B-110), as indicated, inhibits the 1 µM capsaicin (#C-125)-evoked rise in intracellular Ca2+ (control). mTRPV1-C6 cells were loaded with Fluo-3-AM. Changes in intracellular Ca2+ were detected as changes in Fluo-3 fluorescent emission following agonist application.
Alomone Labs BCTC blocks TRPV1 channels expressed in C6 cells.Pre-incubation with 0.1 nM, 1 nM and 10 nM BCTC (#B-110), as indicated, inhibits the 1 µM capsaicin (#C-125)-evoked rise in intracellular Ca2+ (control). mTRPV1-C6 cells were loaded with Fluo-3-AM. Changes in intracellular Ca2+ were detected as changes in Fluo-3 fluorescent emission following agonist application.
- Vriens, J. et al. (2009) Mol. Pharmacol. 75, 1262.
- McLeod, R.L. et al. (2006) Cough 2,10.
- Horton, J.S. et al. (2013) Channels (Austin) 7, 17.
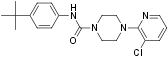
BCTC (N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide) is a potent, selective antagonist of the vanilloid receptor 1 (TRPV1) that is orally bioavailable. BCTC has the ability to block the activation of TRPV1 by capsaicin and by low pH. It has an IC50 of 35 nM3. Oral administration of BCTC in rats significantly reduces mechanical and thermal hyperalgesia associated with inflammation or nerve injury1.
TRPV1 is a member of a distinct subgroup of the transient receptor potential (TRP) ion channel family, highly expressed in primary nociceptors. TRPV1 can be activated by several different stimuli such as heat, acid, certain arachidonic acid derivatives and direct phosphorylation via PKC. TRPV1 may serve a central role in inflammatory nociception2.
Several recent studies have showed that administration of BCTC in patients suffering from symptoms of cardiac hypertrophy and heart failure can overcome loss-of-heart function.
It has been suggested that TRPV1 blockade can be used as a new treatment strategy for cardiac hypertrophy and heart failure3.
BCTC (#B-110) is a highly pure, synthetic, and biologically active compound.
Applications
Citations
- HEK 293 transfected cells.
McArthur, J.R. et al. (2019) Br. J. Pharmacol. 176, 2264.